A Phase IV Clinical Study to Evaluate the Efficacy and Safety of Bresol and Septilin Tablets in Combination for Chronic Allergic Rhinitis and Recurrent Bacterial Sinusitis along with Impact on Immune System
1Professor and HOD of ENT, Kempegowda Institute of Medical Sciences, Bangalore, India
2Consultant Otorhinolaryngologist, Gowri Prasanna Clinic, Bengaluru, India
3Principal Scientist, The Himalaya Drug Company, Makali, Bengaluru, India
- *Corresponding Author:
- Dr. Palaniyamma D, MBBS, MD
Principal Scientist, R&D
The Himalaya Drug Company Makali
Bengaluru 562 162, India
Tel: +918067549920
E-mail: dr.palani@himalayawellness.com
Citation: ZSomashekara M, et al. A Phase IV Clinical Study to Evaluate the Efficacy and Safety of Bresol and Septilin Tablets in Combination for Chronic Allergic Rhinitis and Recurrent Bacterial Sinusitis along with Impact on Immune System. Ann Med Health Sci Res. 2020;10: 912-921.
This is an open access article distributed under the terms of the Creative Commons Attribution‑NonCommercial‑ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non‑commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
The aim of the study was to evaluate the efficacy and safety of Bresol and Septilin tablets in combination in the management of chronic allergic rhinitis and recurrent bacterial sinusitis. A Phase IV clinical study was conducted to provide symptomatic relief in chronic allergic rhinitis and recurrent bacterial sinusitis along with impact on Immune System in 200 subjects. Subjects were assessed for all the details as per the inclusion and exclusion criteria. All the subjects were screened, as per the protocol and the eligible subjects were enrolled in the study and were subjected to study specific safety and clinical assessment, Hematological and biochemical investigations, recording adverse events during assessment visits at Day 30, 60 and 90. This study observed a significant symptomatic reduction in the mean scores for sneezing, nasal congestion, itching of the eyes, itching of nose, postnasal drip, rhinorrhea, headache, cough, and wheezing, nasal obstruction, watery eyes in both adults as well as in pediatric subjects with Bresol and Septilin tablets treatment in combination at recommended doses.
Keywords
Allergic rhinitis; Recurrent Bacterial sinusitis; Postnasal drip; Rhinorrhea
Introduction
Our body plays a host to a number of microorganisms and can be invaded at times by the outer microorganisms [called infection]. Among various infections, respiratory tract infections (RTIs) are common in patients of all ages and are associated with high morbidity and high rates of medical consultations. Respiratory tract infections can be divided into upper and lower respiratory tract infections More than 10% of all young children suffer from recurrent upper and lower respiratory tract infections. [1,2] Upper respiratory infections (URI) are commonly complicated with bacterial infections, mostly with acute otitis media (29% of the cases in children) or followed by acute bacterial sinusitis (ABS) [5-8%] of the cases in children and 0.5-2% in adults. [3,4] Although not usually life-threatening in adults, these infections represent a major medical concern in terms of high morbidity and absenteeism from work.
Allergic rhinitis is the inflammation of the mucous membrane of nasal passage caused by allergens such as dust mites, fecal particles, cockroach residues pets, pests, and some molds. Sensitization to inhaled allergens begins during the first year of life and since infections tend to occur in children more frequently due to developing immune system in first 2-3 years of life and Bacterial sinusitis is the inflammation of sinuses and inflammation occurring in both nasal passage and sinus occurring together is called as rhinosinusitis. Rhinosinusitis can be of acute, subacute and chronic type. Acute rhinosinusitis is a condition which lasts up to 4 weeks. [5] Sub-acute rhinosinusitis lasts from 4-12 weeks and chronic is of >12 weeks duration.
Allergic rhinitis and Bacterial sinusitis both are caused by IgE-mediated reactions against inhaled allergens and involves mucosal inflammation by type 2 helper T (Th2) cells type in nature and release cytokines (e.g., interleukin [IL]-3, IL-4, IL-5, and IL-13) that promote immunoglobulin E (IgE) production by plasma cells. Increase in the immunological markers (IgE, IL-4 and INF) are seen in both allergic rhinitis and recurrent bacterial sinusitis.
With the limitations and side effects of conventional therapies such as Antihistamines which are useful in controlling some of the symptoms (sneezing, rhinorrhea and pruritus) of allergic rhinitis, but they are less effective in relieving the nasal obstruction and ocular symptoms. Sympathomimetic agents stimulate alpha-receptors and reduce the oedema of the nasal mucous membranes in allergic rhinitis, but these drugs may induce elevated blood pressure, nervousness and insomnia. Intranasal corticosteroid injections also have major adverse effects such as adrenal suppression, which may lead to transient or permanent loss of vision Systemic corticosteroids are an inappropriate therapy for patients with mild to moderate allergic rhinitis; [6,7] a novel herbal formulation is of high need, which can serve to reduce the underlying pathology of allergic rhinitis
and bacterial sinusitis by enhancing the immunity and prevent the recurrence of episodes of allergic rhinitis and bacterial sinusitis. Hence, a unique polyherbal formulation of two tablets like Bresol and Septilin were given in combination, having potent herbal actives known to serve the above mentioned purpose with enhanced immunity was considered. This study was conducted to clinically evaluate the safety and efficacy of Bresol and Septilin tablets in both adult as well as in pediatric subjects with allergic rhinitis and recurrent bacterial sinusitis.
Aim of the Study
The aim of the study was to evaluate the efficacy and safety of Bresol and Septilin tablets in combination in chronic allergic rhinitis and recurrent bacterial sinusitis along with impact on immune system.
Materials and Methods
Inclusion criteria
Subjects of either gender aged between 8 to 17 years and 18 to 60 years with symptoms of chronic allergic rhinitis, Subjects of either gender aged between 8 to 17 years and 18 to 60 years with symptoms of recurrent bacterial sinusitis, Subjects who are willing to give a written informed consent and follow the schedule.
Exclusion criteria
A known history or present condition of allergic response to any ingredients in the products, Individuals on systemic treatment with corticosteroids or immunosuppressive drugs, immuno compromised individuals, severe hepatic & renal failure, pregnant or lactating women.
Study procedure
Before entering into the study, the subjects were prescreened by the investigator for the criteria indicated in the Subject Selection section. Only subjects who met the requirements of this section and were willing to sign an informed consent form with an updated medical history on file with the investigator, were enrolled into the study.
A written consent was obtained from each of the subjects after confirming their total fitness to participate in the study. Adult subjects were advised to take two tablets each of Bresol and Septilin tablets twice daily oral for a period of 90 days. Pediatric subjects were advised to take one tablet each of Bresol and Septilin twice daily oral for a period of 90 days. Identification numbers were given to all patients. All the subjects were assessed for clinical parameters at baseline, day 30, day 60 and day 90. Quality of life was assessed at baseline and the end of the study. Immunological parameters [Immunoglobulin (IgE), Interleukins (IL-4) and Interferons (IFN)] were also assessed at screening and the end of study. All the subjects were followedup for safety evaluations.
Statistical analysis
All subjects who completed the trial were included in the analysis of the data. The data was stratified by age (younger <18 up to 8 years and adult >18 years up to 60years) and the data was expressed as descriptive statistics (Mean, Standard deviation). For safety, parameters like Hematology, Descriptive statistical analysis was done and data represented in Mean ± SD. Analysis of primary efficacy parameters was performed using the Kruskal-Wallis test followed by Dunn’s multiple comparisons test with two-sided significance level of 0.05. Quality of Life questionnaire was analyzed by using Wilcoxon matched-pair signed rank test. Descriptive statistics was provided for all safety and efficacy parameters, Values were represented in Mean ± SD, number of subjects and percentages, significance was fixed at p<0.05, two tailed p value. All statistical analyses were done by using GraphPad prism software Version 6.07 for Windows, GraphPad Software, San Diego, California, USA.
Results
There were 200 subjects enrolled into the study. All subjects (both adult and pediatric) were given Bresol and Septilin tablets. All the 200 subjects completed the study and were considered for efficacy evaluation (Clinical parameters) at baseline, day 30, day 60 and at day 90. Total 200 subjects were considered for statistical evaluation.
Assessment on demographic data in pediatric subjects
Among 200 subjects, 27 were pediatric subjects with an average age of 12.6 ± 3.3 out of which 7 were female and remaining 20 were male subjects. Among 27 pediatric subjects, all had allergic rhinitis and only 10 subjects were associated with sinusitis [Table 1].
| No Subjects <18 | 27 out of 200 |
| Age in Years (Mean ± SD) | 12.6 ± 3.3 |
| Minimum Age | 8 |
| Maximum Age | 17 |
| Gender | |
| Male | 20 |
| Female | 7 |
| Subject classification of allergic Rhinitis. | 27 |
| Associated sinusitis | 10 |
Table 1: Demographics data of pediatric subjects (n=27).
Symptomatic assessment in pediatric subjects
The score for sneezing was 4.74 ± 1.13 at entry which got reduced to 1.11 ± 0.93 at the end of the study (90 days) with significance of p<0.0001 as compared to baseline. The score for nasal congestion was 4.3 ± 1.64 at entry which got reduced to 1.3 ± 0.95 at the end of the study (90 days) with a significance of p<0.0001 as compared to baseline. The score for Itching of the eyes was 0.93 ± 1.84 at entry which reduced to 0.11 ± 0.32 at the end of the study (90 days) with no statistical significance. The score for Itching of the nose was 0.5 ± 1.07 at entry which reduced to 0.04 ± 0.2 at the end of the study (90 days) with no statistical significance. The score for Post-nasal drip was 1.56 ± 1.95 at entry which reduced to 0.22 ± 0.64 at the end of the study (90 days) with no statistical significance as compared to baseline. The score for Rhinorrhea was 4.63 ± 1.45 at entry which reduced to 1.33 ± 1.11 at the end of study (90 days) with a significance of p<0.0001 as compared to baseline. The score for headache was 1.73 ± 2.33 at entry 0.65 ± 1.02 at the end of the study (90 days) with a significance of p<0.0079 as compared to baseline. The score for Cough was 1.41 ± 1.67 at entry which reduced to 0.41 ± 0.64 at the end of the study (90 days) with a significance of p<0.001 as compared to baseline. The score for wheezing was 0.67 ± 1.57 at entry which reduced 0.22 ± 0.64 at the end of the study (90 days) with no statistical significance. The score for nasal obstruction was 4.74 ± 0.94 at entry which reduced to 1.74 ± 0.94 at the end of the study (90 days) with a significance of p<0.0001 as compared to baseline. The score for watery eyes was 0.63 ± 1.36 at entry which reduced to 0.04 ± 0.19 at the end of the study (90 days) with no statistical significance. The score for daily rhinitis symptom score was 26.19 ± 12.29 at entry which reduced to 7.26 ± 4.86 at the end of the study (90 days) with a significance of p<0.0001 as compared to baseline [Table 2].
| Symptoms | Baseline | 30 days | 60 days | 90 days | |||
|---|---|---|---|---|---|---|---|
| Mean + SD | 4.74 + 1.13 | 3.56 + 1.12 | 2.44 + 1.09 | 1.11 + 0.93 | |||
| Sneezing | p value | a:p<0.046 | a:p<0.0001 | a:p<0.0001 | |||
| Median | 5 | 4 | 2 | 1 | |||
| Minimum | 3 | 2 | 1 | 0 | |||
| Maximum | 6 | 5 | 4 | 3 | |||
| Nasal congestion | Mean ± SD | 4.3 ± 1.64 | 3.15 ± 1.41 | 2.15 ± 1.13 | 1.3 ± 0.95 | ||
| p value | a:p<0.0342 | a:p<0.0001 | a:p<0.0001 | ||||
| Median | 4 | 3 | 2 | 1 | |||
| Minimum | 0 | 0 | 0 | 0 | |||
| Maximum | 6 | 5 | 4 | 3 | |||
| Itching of the eyes | Mean ± SD | 0.93 ± 1.84 | 0.52 ± 1.22 | 0.3 ± 0.78 | 0.11 ± 0.32 | ||
| Median | 0 | 0 | 0 | 0 | |||
| Minimum | 0 | 0 | 0 | 0 | |||
| Maximum | 6 | 4 | 3 | 1 | |||
| Itching of the nose | Mean ± SD | 0.5 ± 1.07 | 0.27 ± 0.6 | 0.12 ± 0.43 | 0.04 ± 0.2 | ||
| Median | 0 | 0 | 0 | 0 | |||
| Minimum | 0 | 0 | 0 | 0 | |||
| Maximum | 3 | 2 | 2 | 1 | |||
| Post-nasal drip | Mean ± SD | 1.56 ± 1.95 | 1 ± 1.44 | 0.52 ± 0.94 | 0.22 ± 0.64 | ||
| Median | 0 | 0 | 0 | 0 | |||
| Minimum | 0 | 0 | 0 | 0 | |||
| Maximum | 7 | 6 | 4 | 3 | |||
| Rhinorrhea | Mean ± SD | 4.63 ± 1.45 | 3.3 ± 1.38 | 2.3 ± 1.24 | 1.33 ± 1.11 | ||
| p value | a:p<0.0216 | a:p<0.0001 | a:p<0.0001 | ||||
| Median | 5 | 3 | 2 | 1 | |||
| Minimum | 0 | 0 | 0 | 0 | |||
| Maximum | 7 | 5 | 4 | 4 | |||
| Headache | Mean ± SD | 1.73 ± 2.33 | 1.27 ± 1.73 | 0.96 ± 1.37 | 0.65 ± 1.02 | ||
| p value | a:p<0.0079 | ||||||
| Median | 0 | 0 | 0 | 0 | |||
| Minimum | 0 | 0 | 0 | 0 | |||
| Maximum | 6 | 5 | 4 | 3 | |||
| Cough | Mean ± SD | 1.41 ± 1.67 | 0.93 ± 1.24 | 0.67 ± 1.04 | 0.41 ± 0.64 | ||
| p value | a:p<0.0184 | a:p<0.0012 | |||||
| Median | 0 | 0 | 0 | 0 | |||
| Minimum | 0 | 0 | 0 | 0 | |||
| Maximum | 6 | 5 | 4 | 2 | |||
| Wheezing | Mean ± SD | 0.67 ± 1.57 | 0.48 ± 1.25 | 0.37 ± 1.01 | 0.22 ± 0.64 | ||
| Median | 0 | 0 | 0 | 0 | |||
| Minimum | 0 | 0 | 0 | 0 | |||
| Maximum | 6 | 5 | 4 | 3 | |||
| Nasal obstruction | Mean ± SD | 4.74 ± 0.94 | 3.44 ± 1.09 | 2.52 ± 1.05 | 1.74 ± 0.94 | ||
| p value | a:p<0.0095 | a:p<<0.0001 | a:p<<0.0001 | ||||
| Median | 5 | 3 | 2 | 2 | |||
| Minimum | 3 | 2 | 1 | 0 | |||
| Maximum | 6 | 5 | 4 | 4 | |||
| Watery eyes | Mean ± SD | 0.63 ± 1.36 | 0.41 ± 1.01 | 0.15 ± 0.53 | 0.04 ± 0.19 | ||
| Median | 0 | 0 | 0 | 0 | |||
| Minimum | 0 | 0 | 0 | 0 | |||
| Maximum | 5 | 4 | 2 | 1 | |||
| Daily rhinitis symptom score | Mean ± SD | 26.19 ± 12.29 | 18.44 ± 9.81 | 12.56 ± 7.17 | 7.26 ± 4.86 | ||
| p value | a:p<<0.0001 | a:p<<0.0001 | |||||
| Median | 22 | 16 | 11 | 7 | |||
| Minimum | 11 | 7 | 4 | 1 | |||
| Maximum | 63 | 49 | 31 | 17 | |||
a: as compared to Baseline
Significance was fixed at <0.05
Value in: Mean (SD)
Software: GraphPad Prism 6.07
Table 2: Evaluation of efficacy of bresol and septilin tablets in pediatric subjects (n=27).
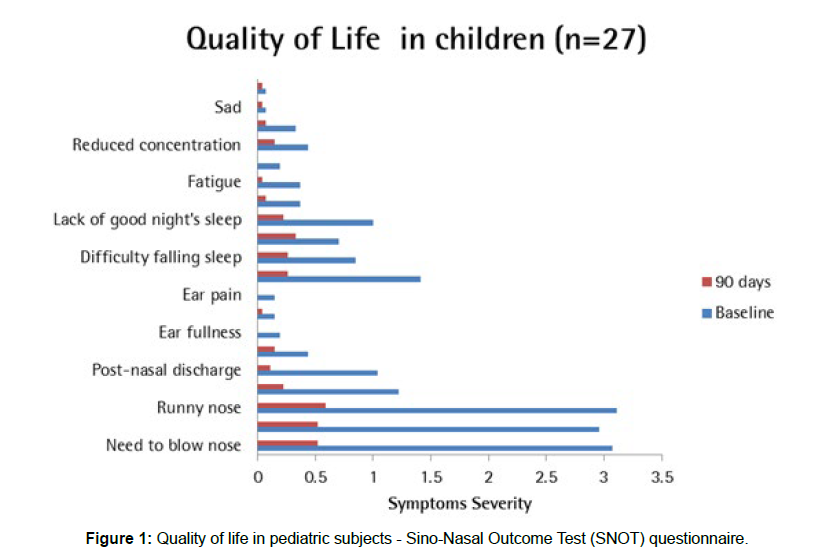
Quality of life assessment in paediatric subjects
Assessment for quality of life (QOL) in pediatric subjects: Assessment of quality of life parameters in pediatric subjects showed reduction in the score for need to blow nose, sneezing, runny nose, cough, post nasal discharge, thick nasal discharge, ear fullness, dizziness, ear pain, facial pain, difficulty falling asleep, wake up at night, lack of good night sleep, waking up tired, fatigue, reduced productivity, reduced concentration, feeling sad, embarrassed and restless as compared to baseline. Total parameters were reduced from 18.15 ± 8.51 at entry to 3.63 ± 3.42 at the end of 90 days with a significance of p<0.0001 [Table 3].
| Parameters | Baseline | 90 days |
|---|---|---|
| Need to blow nose | 3.07 ± 0.73 | 0.52 ± 0.7 |
| a:p<0.0001 | ||
| Sneezing | 2.96 ± 0.81 | 0.52 ± 0.58 |
| a:p<0.0001 | ||
| Runny nose | 3.11 ± 0.75 | 0.59 ± 0.84 |
| a:p<0.0001 | ||
| Cough | 1.22 ± 1.37 | 0.22 ± 0.42 |
| a:p<0.0002 | ||
| Post-nasal discharge | 1.04 ± 1.37 | 0.11 ± 0.32 |
| a:p<0.0006 | ||
| Thick nasal discharge | 0.44 ± 1.01 | 0.15 ± 0.36 |
| Ear fullness | 0.19 ± 0.62 | 0 ± 0 |
| Dizziness | 0.15 ± 0.46 | 0.04 ± 0.19 |
| Ear pain | 0.15 ± 0.46 | 0 ± 0 |
| Facial pain /pressure | 1.41 ± 1.58 | 0.26 ± 0.45 |
| a:p<0.0001 | ||
| Difficulty falling sleep | 0.85 ± 1.1 | 0.26 ± 0.45 |
| a:p<0.0039 | ||
| Wake up at night | 0.7 ± 1.17 | 0.33 ± 0.48 |
| a:p<0.0301 | ||
| Lack of good night's sleep | 1 ± 1.39 | 0.22 ± 0.42 |
| a:p<0.0022 | ||
| Wake up tired | 0.37 ± 0.88 | 0.07 ± 0.27 |
| Fatigue | 0.37 ± 0.84 | 0.04 ± 0.19 |
| a:p<0.0363 | ||
| Reduced productivity | 0.19 ± 0.68 | 0 ± 0 |
| Reduced concentration | 0.44 ± 0.97 | 0.15 ± 0.36 |
| Frustrated/ restless/ irritable | 0.33 ± 0.92 | 0.07 ± 0.27 |
| Feeling Sad | 0.07 ± 0.27 | 0.04 ± 0.19 |
| Embarrassed | 0.07 ± 0.27 | 0.04 ± 0.19 |
| Total | 18.15 ± 8.51 | 3.63 ± 3.42 |
| a:p<0.0001 |
a: as compared to Baseline, Significance was fixed at <0.05,
Value in: Mean (SD), Software: GraphPad Prism 6.07
Table 3: Quality of life in children (n=27).
Assessment of QoL in pediatric subjects through SNOT questionnaire revealed that post 90 days of intervention with Bresol and septilin tablets; there was a significant reduction in the symptoms as compared to the baseline [Figure 1].
Assessment of demographic data in adult subjects: Among 200 subjects, 173 subjects were adult subjects with an average age of 36.1 ± 12.8 and all subjects were diagnosed with allergic rhinitis with only 132 subjects associated with recurrent bacterial sinusitis [Table 4].
| No of Subjects = 18 | 173 out of 200 |
| Age in Years | 36.1 ± 12.8 |
| Minimum Age | 18 |
| Maximum Age | 77 |
| Gender | |
| Male | 108 |
| Female | 65 |
| Subject classification of allergic Rhinitis. | 173 |
| Associated Sinusitis | 132 |
Table 4: Demographics of study population in data of study in adults (n=173).
Symptomatic assessment in adult subjects: The score for sneezing was 5.36 ± 1.47 at entry which reduced to 1.9 ± 1.65 at the end of the study (90 days) with a significance of p<0.0001 as compared to baseline. The score for nasal congestion was 4.54 ± 1.45 at entry which reduced to 1.44 ± 1.16 at the end of the study (90 days) with a significance of p<0.0001 as compared to baseline. The score for Itching of the eyes was 2.59 ± 2.12 at entry which reduced to 0.67 ± 0.92 at the end of the study (90 days) with a significance of p<0.0001 as compared to baseline. The score for Itching of the nose was 2.64 ± 2.04 at entry which reduced to 0.59 ± 0.86 at the end of the study (90 days) with a significance of p<0.0001 as compared to baseline. The score for post-nasal drip was 2.29 ± 1.96 at entry which reduced to 0.51 ± 0.8 at the end of the study (90 days) with a significance of p<0.0001 as compared to baseline. The score for rhinorrhea was 4.37 ± 1.54 at entry which reduced to 1.23 ± 1.37 at the end of the study (90 days) with a significance of p<0.0001 as compared to baseline. The score for headache was 2.82 ± 2.03 at entry which reduced to 0.65 ± 1.02 at the end of the study (90 days) with a significance of p<0.0079 as compared to baseline. The score for cough was 1.41 ± 1.67 at entry which reduced to 0.41 ± 0.64 at the end of the study (90 days) with a significance of p<0.0012 as compared to baseline. The score for wheezing was 0.67 ± 1.57 at entry which reduced to 0.22 ± 0.64 at the end of the study (90 days) with no statistical significance. The score for nasal obstruction was 4.74 ± 0.94 at entry which reduced to 1.74 ± 0.94 at the end of the study (90 days) with a significance of p<0.0001 as compared to baseline. The score for watery eyes was 0.63 ± 1.36 at entry which reduced to 0.04 ± 0.19 at the end of the study (90 days) with no statistical significance. The score for daily rhinitis symptom score was 26.19 ± 12.29 at entry which reduced to 7.26 ± 4.86 at the end of the study (90 days) with a significance of p<0.0001 as compared to baseline [Table 5].
| Daily individual symptoms of rhinitis | Baseline | 30 days | 60 days | 90 days | |
|---|---|---|---|---|---|
| Sneezing | Mean ± SD | 5.36 ± 1.47 | 4.01 ± 1.5 | 2.92 ± 1.53 | 1.9 ± 1.65 |
| p value | a:p<0.0001 | a:p<0.0001 | a:p<0.0001 | ||
| Median | 6 | 4 | 3 | 2 | |
| Minimum | 0 | 0 | 0 | 0 | |
| Maximum | 8 | 7 | 7 | 6 | |
| Nasal congestion | Mean ± SD | 4.54 ± 1.45 | 3.22 ± 1.31 | 2.2 ± 1.24 | 1.44 ± 1.16 |
| p value | a:p<0.0001 | a:p<0.0001 | a:p<0.0001 | ||
| Median | 5 | 3 | 2 | 1 | |
| Minimum | 0 | 0 | 0 | 0 | |
| Maximum | 8 | 6 | 5 | 5 | |
| Itching of the eyes | Mean ± SD | 2.59 ± 2.12 | 1.72 ± 1.58 | 1.12 ± 1.2 | 0.67 ± 0.92 |
| p value | a:p<0.0001 | a:p<0.0001 | a:p<0.0001 | ||
| Median | 3 | 2 | 1 | 0 | |
| Minimum | 0 | 0 | 0 | 0 | |
| Maximum | 8 | 6 | 5 | 5 | |
| Itching of the nose | Mean ± SD | 2.64 ± 2.04 | 1.63 ± 1.53 | 1.02 ± 1.16 | 0.59 ± 0.86 |
| p value | a:p<0.0001 | a:p<0.0001 | a:p<0.0001 | ||
| Median | 3 | 2 | 1 | 0 | |
| Minimum | 0 | 0 | 0 | 0 | |
| Maximum | 8 | 6 | 5 | 5 | |
| Post-nasal drip | Mean ± SD | 2.29 ± 1.96 | 1.4 ± 1.35 | 0.93 ± 1.13 | 0.51 ± 0.8 |
| p value | a:p<0.0005 | a:p<0.0001 | a:p<0.0001 | ||
| Median | 2 | 1 | 0 | 0 | |
| Minimum | 0 | 0 | 0 | 0 | |
| Maximum | 8 | 5 | 4 | 3 | |
| Rhinorrhea | Mean ± SD | 4.37 ± 1.54 | 3.01 ± 1.5 | 2.03 ± 1.48 | 1.23 ± 1.37 |
| p value | a:p<0.0001 | a:p<0.0001 | a:p<0.0001 | ||
| Median | 4 | 3 | 2 | 1 | |
| Minimum | 0 | 0 | 0 | 0 | |
| Maximum | 8 | 7 | 6 | 5 | |
| Headache | Mean ± SD | 1.73 ± 2.33 | 1.27 ± 1.73 | 0.96 ± 1.37 | 0.65 ± 1.02 |
| p value | a:p<0.0079 | ||||
| Median | 0 | 0 | 0 | 0 | |
| Minimum | 0 | 0 | 0 | 0 | |
| Maximum | 6 | 5 | 4 | 3 | |
| Cough | Mean ± SD | 1.41 ± 1.67 | 0.93 ± 1.24 | 0.67 ± 1.04 | 0.41 ± 0.64 |
| p value | a:p<0.0184 | a:p<0.0012 | |||
| Median | 0 | 0 | 0 | 0 | |
| Minimum | 0 | 0 | 0 | 0 | |
| Maximum | 6 | 5 | 4 | 2 | |
| Wheezing | Mean ± SD | 0.67 ± 1.57 | 0.48 ± 1.25 | 0.37 ± 1.01 | 0.22 ± 0.64 |
| Median | 0 | 0 | 0 | 0 | |
| Minimum | 0 | 0 | 0 | 0 | |
| Maximum | 6 | 5 | 4 | 3 | |
| Nasal obstruction | Mean ± SD | 4.74 ± 0.94 | 3.44 ± 1.09 | 2.52 ± 1.05 | 1.74 ± 0.94 |
| p value | a:p<0.0095 | a:p<<0.0001 | a:p<<0.0001 | ||
| Median | 5 | 3 | 2 | 2 | |
| Minimum | 3 | 2 | 1 | 0 | |
| Maximum | 6 | 5 | 4 | 4 | |
| Watery eyes | Mean ± SD | 0.63 ± 1.36 | 0.41 ± 1.01 | 0.15 ± 0.53 | 0.04 ± 0.19 |
| Median | 0 | 0 | 0 | 0 | |
| Minimum | 0 | 0 | 0 | 0 | |
| Maximum | 5 | 4 | 2 | 1 | |
| Daily rhinitis symptom score | Mean ± SD | 26.19 ± 12.29 | 18.44 ± 9.81 | 12.56 ± 7.17 | 7.26 ± 4.86 |
| p value | a:p<<0.0001 | a:p<<0.0001 | |||
| Median | 22 | 16 | 11 | 7 | |
| Minimum | 11 | 7 | 4 | 1 | |
| Maximum | 63 | 49 | 31 | 17 |
a: as compared to Baseline
Significance was fixed at <0.05
Value in: Mean (SD)
Software: GraphPad Prism 6.07
Table 5: Evaluation of clinical parameters (efficacy) of bresol and septilin tablets in adults (N=173).
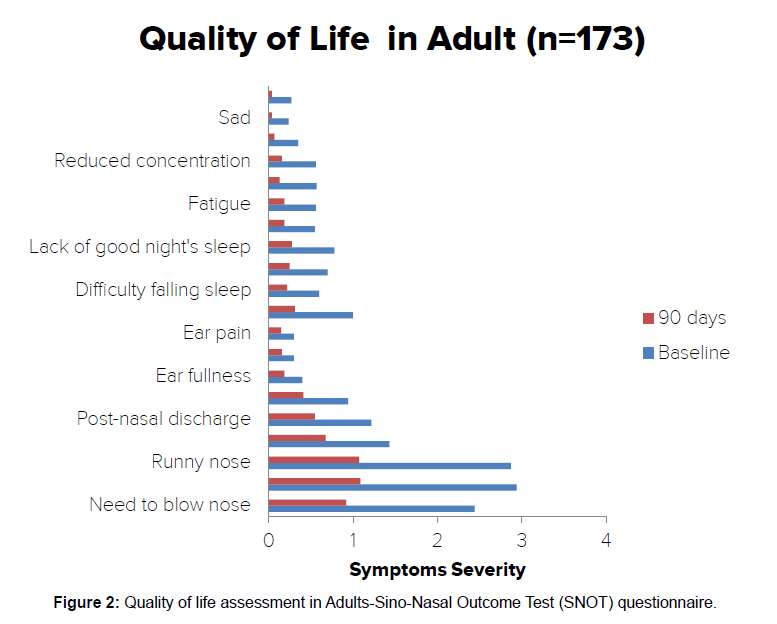
Assessment for quality of life (QOL) in adult subjects: Assessment of quality of life parameters in adult subjects showed reduction in the score for need to blow nose, sneezing, runny nose, cough, post nasal discharge, thick nasal discharge, ear fullness, dizziness, ear pain, facial pain, difficulty falling asleep, waking up at night, lack of good night sleep, waking up tired, fatigue, reduced productivity, reduced concentration, feeling sad, embarrassed and restless as compared to baseline. Total parameters were reduced from 18.98 ± 8.88 at entry to 7.08 ± 5.61 at the end of 90 days with significance of p<0.0001 as compared to baseline [Table 6].
| Parameters | Baseline | 90 days |
|---|---|---|
| Need to blow nose | 2.44 ± 0.98 | 0.92 ± 0.85 |
| a:p<0.0001 | ||
| Sneezing | 2.94 ± 0.77 | 1.09 ± 0.97 |
| a:p<0.0001 | ||
| Runny nose | 2.87 ± 1.01 | 1.07 ± 0.92 |
| a:p<0.0001 | ||
| Cough | 1.43 ± 1.28 | 0.68 ± 0.76 |
| a:p<0.0001 | ||
| Post-nasal discharge | 1.22 ± 1.06 | 0.55 ± 0.7 |
| a:p<0.0001 | ||
| Thick nasal discharge | 0.94 ± 1 | 0.41 ± 0.62 |
| a:p<0.0001 | ||
| Ear fullness | 0.4 ± 0.69 | 0.19 ± 0.43 |
| a:p<0.0036 | ||
| Dizziness | 0.3 ± 0.55 | 0.16 ± 0.4 |
| a:p<0.0119 | ||
| Ear pain | 0.3 ± 0.58 | 0.15 ± 0.37 |
| a:p<0.0004 | ||
| Facial pain /pressure | 1 ± 1.33 | 0.31 ± 0.54 |
| a:p<0.0001 | ||
| Difficulty falling sleep | 0.6 ± 0.96 | 0.22 ± 0.47 |
| a:p<0.0001 | ||
| Wake up at night | 0.7 ± 0.95 | 0.25 ± 0.47 |
| a:p<0.0001 | ||
| Lack of good night's sleep | 0.78 ± 1.09 | 0.28 ± 0.49 |
| a:p<0.0001 | ||
| Wake up tired | 0.55 ± 0.83 | 0.19 ± 0.41 |
| a:p<0.0001 | ||
| Fatigue | 0.56 ± 0.85 | 0.19 ± 0.42 |
| a:p<0.0001 | ||
| Reduced productivity | 0.57 ± 0.95 | 0.13 ± 0.37 |
| a:p<0.0001 | ||
| Reduced concentration | 0.56 ± 0.94 | 0.16 ± 0.88 |
| a:p<0.0001 | ||
| Frustrated/ restless/ irritable | 0.35 ± 0.66 | 0.07 ± 0.26 |
| a:p<0.0001 | ||
| Feeling Sad | 0.24 ± 0.48 | 0.04 ± 0.2 |
| a:p<0.0001 | ||
| Embarrassed | 0.27 ± 0.55 | 0.04 ± 0.2 |
| a:p<0.0001 | ||
| Total | 18.98 ± 8.88 | 7.08 ± 5.61 |
| a:p<0.0001 |
a: as compared to Baseline
Significance was fixed at <0.05
Value in: Mean (SD)
Software: GraphPad Prism 6.07
Table 6: Quality of life assessment in adults (n=173).
Assessment of QOL in adult subjects through SNOT questionnaire revealed that post 90 days of intervention with Bresol and septilin tablets; there was a significant reduction in the symptoms as compared to the baseline [Figure 2].
Assessment for immunological parameters (IgE, IFN & IL- 4): Immunological markers were evaluated in 40 subjects for IL-4 and IFN whereas in 117 subjects were evaluated for IgE. Allergic rhinitis (AR) causes inflammation of the nasal mucosa mainly through immunoglobulin E (IgE) and there are elevated levels of interferons (IFN). Although allergic diseases have been linked to an enhanced Th2 immune response associated with high levels of interleukins (IL) IL-4, IL-5 and IL-13, accumulating evidences demonstrate that a decreased T helper cells (Th1) immune response is also important in the pathogenesis of these diseases, and that interferon-g (IFN-g) could act as a central regulator in this phenomenon. Increased IFN-β response in the Sino nasal mucosa may underlie rhinosinusitis pathogenesis.
Assessment of immunological markers in terms of Mean ± SD: The score for Interferon (IFN) was 211.9 ± 73.01 at entry (pre-treatment) was reduced to 157.2 ± 80.31 at the end of study (post-treatment) with a significance of p<0.0001 as compared to baseline. The score for Interleukin (IL-4) was 15.5 ± 3.79 at entry reduced to 13.14 ± 3.47 at the end of study with a significance of p<0.0001 as compared to baseline. Interleukin-4 is elevated during nasal allergic disease and reduction signifies improvement in allergy. The score for IgE was 356 ± 254.1 at entry which was increased to 453.1 ± 278.1 post treatment with no statistical significance [Table 7].
| IFN (pg/ml) (n=40) | Pre-Treatment | Post-Treatment |
|---|---|---|
| Mean ± SD | 211.9 ± 73.01 | 157.2 ± 80.31 |
| a:p<0.0001 | ||
| Median | 220.70 | 164.00 |
| Minimum | 54.00 | 47.33 |
| Maximum | 337.30 | 320.70 |
| IL-4 (pg/ml) (n=40) | ||
| Mean ± SD | 15.5 ± 3.79 | 13.14 ± 3.47 |
| a:p<0.0001 | ||
| Median | 15.90 | 12.30 |
| Minimum | 9.20 | 9.20 |
| Maximum | 23.40 | 22.20 |
| IgE(IU/ml) (n=117) | ||
| Mean ± SD | 356 ± 254.1 | 453.1 ± 278.1 |
| Median | 345.1 | 412.2 |
| Minimum | 60.28 | 50.9 |
| Maximum | 998 | 994 |
a: as compared to Pre Treatment
Table 7: Assessment on immunological parameters (Mean ± SD).
Assessment of immunological markers in terms of percentage N (%): The summary of score for Interferon (IFN) showed the decreased results in 39 (97%) subjects and 1 (3%) showed increased results. The summary of score for Interleukin (IL-4) showed the decreased results in 38 (95%) subjects and 1 (2.5%) showed increased results and with no change in 1 subject (2.5%). The summary of score for Immunoglobulin (IgE) has been showed decreased results in 45 (38%) subjects and 72 (62%) showed increased results as per Table 8.
| IFN- (pg/ml) (n=40) | No. of Subjects (%) |
|---|---|
| Decreased | 39 (97%) |
| Increased | 1 (3%) |
| IL-4 (pg/ml) (n=40) | |
| Decreased | 38 (95%) |
| Increased | 1 (2.5%) |
| No change | 1 (2.5%) |
| IgE (IU/ml) (n=117) | |
| Decreased | 45 (38%) |
| Increased | 72 (62%) |
Table 8: Assessment of immunological parameters [N (%)]
Effect of Bresol and Septilin in individuals where there was reduction of IgE (IU/ml) (n=45): This was evaluated in total 45 subjects. The evaluation of effect of Bresol and septilin tablets showed reduction in mean IgE number from 509 ± 296.3 [pretreatment] to 295.4 ± 217.8 [post-treatment] with a statistical significance of p<0.0001 as compared to baseline [Table 9].
| Pre Treatment | Post Treatment | |||||
|---|---|---|---|---|---|---|
| N | 45 | 45 | ||||
| Mean ± SD | 509 ± 296.3 | 295.4 ± 217.8 | ||||
| Median | 504.8 | 288.4 | ||||
| Minimum | 95.54 | 50.9 | ||||
| Maximum | 998 | 994 | ||||
| p value | a:p<0.0001 | |||||
a: as compared to Pre treatment
Table 9: Effect of bresol and septilin in individuals where there is reduction of IgE (IU/ml) (n=45).
Discussion
The present interventional clinical study observed significant reduction in the mean symptom score for sneezing, nasal congestion, itching of nose, postnasal drip and runny nose. The increased levels of Immunological parameters specific to allergic rhinitis like Interferon, Interleukin-4 and IgE showed a statistical improvement with Bresol and Septilin treatment.
Scores for Quality of life parameters also improved both in adults as well as in pediatric subjects. Immunological markers –Interleukins, Interferons also showed reduction in their levels.
In various studies, active ingredient of Bresol viz. curcumins - I, II and III (Components of Curcuma longa) [8] have been shown to inhibit chemomediators of inflammation -phospholipase, LO, COX-1 and -2, LT, TX, PG, NO, collagenase, elastase, hyaluronidase, monocyte chemoattractant protein-1, interferoninducible protein, TNF-α, and IL-12. [9,10] Curcumins significantly inhibits the production of IL-12, reduces induction of γ-IFN, IL-4 in CD4+ T-lymphocytes by macrophages, leading to the inhibition of T-helper cells-1 cytokine profile (γ- INF and IL-4 production) in CD4+ T-cells. [11]
Gingerols and diarylhepatanoids, the principle active ingredients of Zingiber officinale are potent inhibitors of PG synthetase enzyme and 5-LOX enzymes. Potent inhibition of biotransformation of AA (comparable to indomethacin) by Zingiber officinale was established in one of the study. [12]
Ocimum sanctum has an immuno-stimulatory effect on the humoral immunologic response (an increase in antibody titer), as well as of the CMI response (E-rosette formation and lymphocytosis). [13]
Adhatoda vasica possess potent anti-allergic activity.
Results of the study showed that the potent anti-inflammatory activity of Adhatoda vasica was equivalent to that of hydrocortisone. [14]
Septilin possesses immunomodulatory and anti-inflammatory properties that potentiate the non-specific immune responses of the body. Septilin stimulates phagocytosis by macrophage activation, increases the polymorphonuclear cells and helps overcome infection. Septilin builds up resistance to infection and prevents reinfection. Septilin’s stimulatory effect on the humoral immunity increases the antibody forming cells, thereby increasing the secretion of antibodies into the circulation.
Commiphora mukul showed a wide range of inhibiting activity against both Gram positive and Gram negative bacteria. [15] Maharasnadi quath has analgesic, anti-phologistic and antipyretic properties.
Tinospora cordifolia reverses chemically-induced immunosuppression providing immunomodulatory support. Tinospora cordifolia has potent immunomodulatory and immunostimulatory activities, which increases the levels of antibodies and activate macrophages. [16,17] Rubia cordifolia has immunomodulatory effect which occurs through suppression of iNOS protein.
Emblica officinalis has immunomodulatory and anti-bacterial activity against test bacteria Glycyrrhiza glabra has antiinflammatory action similar to hydrocortisone and other corticosteroid hormones. It also enhances immunostimulation, [18] the essential oil from Saussurea lappa root exhibits strong antiseptic and disinfectant activity against Streptococcus and Staphylococcus. The root shows astringent and antiseptic activity. Shankha bhasma has anti-oxidant action due to its cytoprotective activity in the gastrointestinal tract and reduces gastric irritation. [19]
Study conducted on Bresol tablets by The Himalaya Drug Company, revealed that Bresol tablets along with the improvement in the concerned laboratory findings (TLC, eosinophil count, AEC count), it also prevented recurrence of allergic rhinitis episodes during the entire study period. [20,21]
Studies conducted on septilin tablets revealed the sterilizing effect on the organism with acute rhino-sinusitis where clinical improvement precedes the stage of bacteriological sterility [22] with long-lasting positive quiescence effect on respiratory tract infection and preventive effect on common cold and acute rhinitis. [23]
This study observed a significant reduction in the mean scores for sneezing, nasal congestion, itching of the eyes, itching of nose, postnasal drip, rhinorrhea, headache, cough, wheezing, nasal obstruction, watery eyes in both adults as well as pediatric subjects with the administration of Bresol-Septilin tablets.
The quality of life parameters in both adults and pediatric subjects showed good improvement from the baseline to the end of study making this combination of dru23g therapy In IgE analysis, there was a positive trend suggestive of combined use of Bresol and Septilin Tablets benefit in the target population. With Bresol and Septilin tablets as valuable tool in the management of both allergic rhinitis and bacterial sinusitis. The elevated levels of biomarkers of gamma interferons (γ-IFN) and IL-4 reduced significantly in chronic allergic subjects with the combined use of Bresol and Septilin tablets.
Conclusion
From the present clinical study, it can be concluded that the beneficial results could be possibly due to the synergistic potential effects of the ingredients of Bresol and Septilin tablets. With these observations it can be summarized that Bresol and Septilin treatment brings favourable benefit in significantly improving the clinical symptoms, improvement in specific immunological parameters as well as quality of life in both adult as well as in pediatric subjects suffering from chronic allergic rhinitis and recurrent bacterial sinusitis.
Competing Interests
The authors declare that they have no competing interests.
References
- Hak E, Rovers MM, Sachs AP, Stalman WA, Verheij TJ. Is asthma in 2–12 year-old children associated with physician-attended recurrent upper respiratory tract infections?. Eur J Epidemiol. 2003;18:899-902.
- Niemelä M, Uhari M, Möttönen M. A pacifier increases the risk of recurrent acute otitis media in children in day care centers. Pediatrics. 1995;96:884-888.
- Wald ER, Guerra N, Byers C. Upper respiratory tract infections in young children: duration of and frequency of complications. Pediatrics. 1991;87:129-133.
- Gwaltney Jr JM, Wiesinger BA, Patrie JT. Acute community-acquired bacterial sinusitis: The value of antimicrobial treatment and the natural history. Clin Infect Dis. 2004;38:227-233
- Rosenfeld RM, Andes D, Bhattacharyya N. Clinical Practice Guideline: Adult sinusitis. Otolaryngol Head Neck Surg. 2007;137:S1-31.
- Blackwell GJ, Carnuccio R, Di Rosa M, Flower RJ, Parente L, Persico P. Macrocortin: A polypeptide causing the anti-phospholipase effect of glucocorticoids. Nature. 1980;287:147-149.
- Siegel SC. Topical corticosteroids in the management of rhinitis. In: Rhinitis. Settipane, G.A. (Editor), 2nd edition, Providence, Rhode Island: Oceanside Publications, USA. 1991:231-234.
- Kim JE, Kim AR, Chung HY, Han SY, Kim BS, Choi JS. In vitro peroxynitrite scavenging activity of diarylheptanoids from Curcuma longa. Phytother. Res. 2003;17:481-484.
- Chainani Wu N. Safety and anti-inflammatory activity of curcumin: A component of turmeric (Curcuma longa). J Altern Complement Med. 2003;9:161-168.
- Hong CH, Hur SK, Oh OJ, Kim SS, Nam KA, Lee SK. Evaluation of natural products on inhibition of inducible Cyclooxygenase (Cox-2) and Nitric oxide synthase (iNOS) in cultured mouse macrophage cells. J Ethnopharmacol. 2002;83:153-159.
- Kang BY, Song YJ, Kim KM, Choe YK, Hwang SY, Kim TS. Curcumin inhibits Th1 cytokine profile in CD4+ T-cells by suppressing Interleukin-12 production in macrophages. Br J Pharmacol. 1999;128:380-384.
- Kiuchi F, Iwakami S, Shibuya M, Hanaoka F, Sankawa U. Inhibition of prostaglandin and leukotriene biosynthesis by gingerols and diaryl hepatanoids. Chem Pharm Bull. 1992;40:387-391
- Godhwani S, Godhwani JL, Wasum DS. Ocimum sanctum - A preliminary study evaluating its immunoregulatory profile in albino rats. J Ethnopharmacol. 1988;24:193-198.
- Chakraborty A, Brantner AH. Study of alkaloids from Adhatoda vasica on their anti-inflammatory activity. Hytother Res. 2001;15:532-534.
- Saeed MA, Sabir AW. Antibacterial activities of some constituents from oleo-gum-resin of Commiphora mukul. Fitoterapia 2004;75:204-208.
- Manjrekar PN, Jolly CI, Narayanan S. Comparative studies of the immunomodulatory activity of Tinospora cordifolia and Tinospora sinensis. Fitoterapia 2000;71:254-257
- Kapil A, Sharma S. Immunopotentiating compounds from c. J Ethnopharmacol 1997;58:89-95.
- Khare CP. Encyclopedia of Indian Medicinal Plants. Springer. Germany. 2007:317-318
- Mishra S. Shankha bhasma. Ayurvediya Rasashastra. Chaukhamba orientalia, Varanasi, 7th edition, 1997:688.
- Kumar NS, Kolhapure SA. Evaluation of efficacy and safety of HK-07 tablets in allergic rhinitis. Int J Clin Pract. 2004;12:51-61.
- Chandrasekharan S, Paramesh R. Bresol tablets in the management of nasal allergy and allergic rhinitis: An open, prospective trial. Indian J Clin Pract. 2010;21:13-18.
- Cooper RAF. Indigenous combination in the treatment of rhino-sinal infections and sinusitis, Indian J Otolaryngol. 1958;4.
- Mohapatra P, Mohapatra SC, Gupta JN. Clinical quiesence matrix of septilin in respiratory tract infection by indigenous medicine. Indian J Prev Soc Med. 2002;33:42-46.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.