A Telfairia Occidentalis Seed-incorporated Diet May Be Useful in Inhibiting the Induction of Experimental Andropause
- *Corresponding Author:
- Dr. Chukwunonso ECC Ejike
Department of Biochemistry, College of Natural and Applied Sciences, Michael Okpara University of Agriculture, Umudike, PMB 7267, Umuahia, Abia State, Nigeria.
E-mail: ejike.nonso@mouau.edu.ng
Abstract
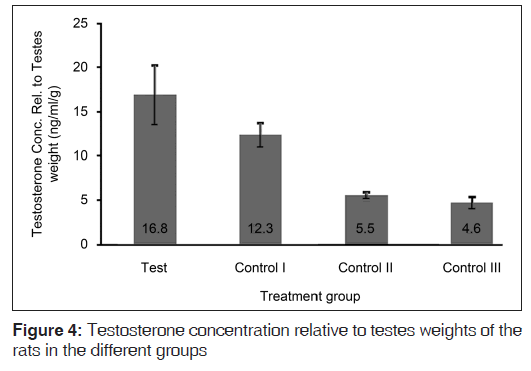
Background: Andropause, a prevalent pathology of men, results from an imbalance in steroid hormone concentrations that often is associated with aging, and reduces the quality of life of the sufferer. This study investigates the usefulness of a diet containing 15% Telfairia occidentalis seeds in the inhibition of the induction of experimental andropause. Materials and Methods: Twenty male rats were divided into four equal groups. Rats in the test group received dihydrotestosterone and estradiol valerate (ratio 10:1) subcutaneously every other day for 28 days and were placed on the test diet. Those in control I received the hormones, but not the test diet. Rats in controls II and III received olive oil (vehicle) and were placed on the test diet and normal diet, respectively. Testes weights and relative weights, serum testosterone concentrations, and testosterone concentration per gram of testicular tissue were measured or determined in all rats using standard protocols. Data were analyzed and differences between means separated using one-way analysis of variance. Results: Rats in the test group had slightly larger mean relative testes weights compared to those in control I, though both were significantly (P<0.001) smaller than the values obtained in controls II and III, respectively. Rats in the test group had significantly higher (P=0.034) serum testosterone concentrations relative to the control I group 6.9(0.3) ng/ml vs. 4.7(0.1) ng/ml, while the testosterone relative to testes weight values (ng/ml/g) of the test group was 16.8(3.4), and for controls I, II, and III the values were 12.3(1.4), 5.5(0.4), and 4.6(0.7), respectively. The differences between the test and control groups were all significant (P=0.04 in control I, and <0.001 in controls II and III). Conclusion: The test diet resulted in a modest reduction of biochemical castration and an improvement in secretory capacity of the testes of the test rats, relative to the control group that received the hormones but was placed on a normal diet. T. occidentalis seeds-incorporated diet may be useful in inhibiting the induction of experimental andropause.
Keywords
Andropause, Biochemical castration, Hormones, Telfairia occidentalis, Testes
Introduction
Andropause, also referred to as late onset hypogonadism (LOH) in males, partial androgen deficiency of aging males (PADAM), or menopause of males, results from an imbalance in the concentrations of the steroid hormones, testosterone and estradiol. It is a process that begins gradually and over time results in both physiologic and psychologic changes.[1] In most men undergoing andropause, the hormonal changes are either an increase in the level of estradiol or a decrease in the level of testosterone, or both[2] – factors that are typical of the effect of biochemical castration – such that serum total, free, or bioavailable testosterone levels drop markedly.[3] Men undergo these hormonal alterations with age and this is worrisome as low testosterone levels are associated with pathologies like diabetes,[4] reduced bone and muscle mass,[5] impotence and impaired sexual function,[6] depression, lethargy, and decreased quality of life.[1]
O’Donnell et al.[7] reported that andropause affects 20% of males over 40 years of age. An earlier study had estimated that 20% of males older than 60 years and 50% of men older than 80 years have andropause.[8] Tariq et al.[9] reported that between 3 and 5 million males in the United States are andropausal. Though such statistics are sparse or outrightly non-existent in Nigeria, it is clear that andropause is a serious and prevalent health care challenge of aging men.
It is, however, important to distinguish andropause due to aging itself from andropause due to an aggregation of the numerous conditions prevalent among, but not restricted to, older adults, called para-aging phenomena.[10] The usefulness of this distinction is that whereas aging is inexorable, para-aging phenomena open up vistas of possible preventive measures aimed at slowing down declines in the health of individuals as they age.[11]
Treatment options for andropause are limited, probably because andropause has only recently been discussed publicly and studied scientifically.[1] Testosterone replacement often by injection or topically has been used to treat andropause but raises concerns especially in men with latent cancer of the prostate or benign prostatic hyperplasia.[12] Given the global drive toward the discovery of food-based drugs for the management of ailments, this study evaluated the usefulness of the seeds of fluted pumpkin (Telfairia occidentalis Hook f.) in the inhibition of biochemical castration, the focal point in andropause pathogenesis, induced by the exogenous administration of steroid hormones, using the rat model.
Materials and Methods
Diet formulation
Mature fluted pumpkin fruits were sourced from a local market, Ahia Ndoru, in Abia state, Nigeria. The seeds were harvested from within the fruits, cleaned, and shelled manually. Good seeds (after careful sorting) were boiled for 1 hour in tap water, then dried in an oven at 40°C to a constant weight, and thereafter milled in a laboratory miller. A diet containing 15% of the boiled, oven-dried, and milled seeds and 75% normal rat chow was prepared. The diet was homogenized thoroughly and pelletized manually. The pellets were dried in an oven at 40°C (to a constant weight) and thereafter cooled and stored in air-tight containers until they were used as test diet. Normal homogenized and manually pelletized, oven-dried rat chow was used as control diet in the relevant groups.
Rats
Twenty 1-month-old male Wistar rats were obtained from the Animal Breeding Unit of the Faculty of Veterinary Medicine, University of Nigeria, Nsukka. On arrival, the rats were acclimatized to our animal house for 1 week before being randomly assigned to four cages/groups. The rats were exposed to approximately 12-hour light/dark cycles under humid tropical conditions, given tap water and feed ad libitum, and housed in standard plastic cages (five per cage) throughout the 28-day duration of the study.
Experimental design
Rats in the test group received 9 mg/kg body weight dihydrotestosterone (DHT) + 0.9 mg/kg body weight estradiol valerate as subcutaneous injections every other day for 28 days and were concomitantly placed on the test diet. Rats in the first control group (control I) were given the hormones akin to those in the test group, but received a normal diet in place of the test diet. Rats in the second control group (control II) received subcutaneous olive oil (vehicle) for the same period, and like the test group, were placed on the test diet. Rats in the third control group (control III) were treated like those in control II, but were placed on a normal diet. Rats in all the groups had mean weights that were statistically similar at the start of the study.
Determinations
After 28 days, the rats were anaesthetized by a brief exposure to trichloromethane vapor and bled by cardiac puncture. The sera were carefully separated and used for the determination of testosterone concentration. Each rat’s carcass was promptly dissected and the testes were carefully excised and freed of external fascias, washed in cold normal saline, blotted with filter paper, and weighed on a sensitive balance. The mean weight of both testes per rat was recorded. The relative weight of the testes was determined by dividing the mean weight of the rat testes with the whole body weight of the respective rat just before sacrifice.
A solid-phase enzyme immunoassay (EIA) quantitative method was employed for the determination of the concentration of testosterone in serum. The protocol was based on the method of Turkes et al.[13] and involved the competition of testosterone in serum and enzyme-labeled testosterone for binding with antitestosterone antibody immobilized on the microwell surface. After color development, the absorbances of the samples were read in a microwell reader and the concentration of testosterone in each sample was determined by extrapolation from a standard curve.
Testosterone relative to testes weight values was determined by dividing the concentration of testosterone per sample by the mean weight of the testes of the rat from which the serum sample was taken ab initio.
Data analysis
Data got from the study were subjected to descriptive statistics and the results presented as means±standard deviations in bar charts. Differences between means were separated by one-way analysis of variance (ANOVA), followed by post hoc multiple comparisons, with the least significant threshold employed at P≤0.05. Data analysis was done using the statistical software package SPSS for windows version 17.0 (SPSS Inc., Chicago, IL, USA).
Results
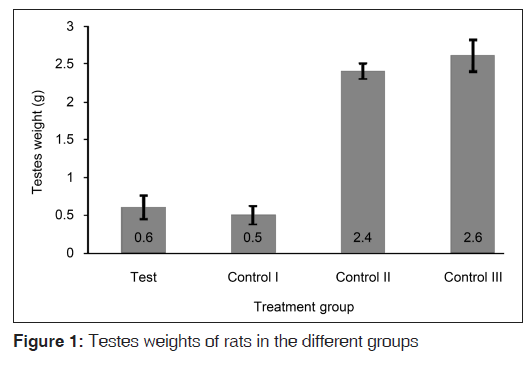
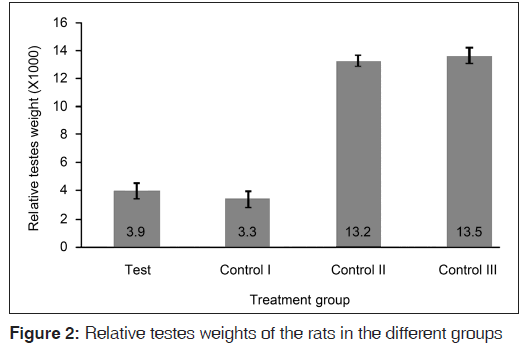
The weights of the testes of rats that received the hormones (test and control I groups) were clearly smaller than those of the other control groups that did not receive the hormonal challenge [Figure 1]. The mean testes weight of the rats in the test group was significantly smaller than those of the rats in the control II (P<0.001) and control III (P<0.001) groups, but statistically similar to that of the rats in the contro I group (P=0.400). The relative testes weights of the rats followed the same trend [Figure 2], though the rats in the test group had a more obviously larger (albeit statistically insignificant (P=0.199)) relative testes weight compared to that of their counterparts in the control I group.
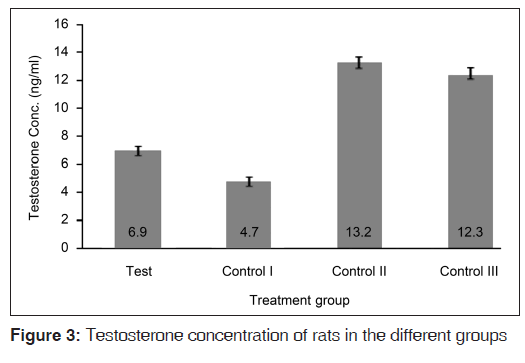
Figure 3 shows that the serum testosterone concentrations were copiously higher in the control II and control III groups (that did not receive the hormones) relative to the test and control I groups. The test diet resulted in rats in the test group having significantly higher (P=0.034) serum testosterone concentrations relative to the control I group. However, the serum testosterone concentrations of rats in the test group were still significantly lower than those of the control II (P<0.001) and control III (P<0.001) groups. In Figure 4, it is seen that the testosterone relative to testes weight values were clearly higher in the hormonally challenged groups, relative to their counterparts that were spared of the exogenous hormones. The test diet resulted in testosterone relative to testes weight values that were significantly higher than values from all the control groups (P=0.045 for control I; P<0.001 for both controls II and III).
Discussion
The seeds of T. occidentalis Hook f. have been shown to possess excellent nutritive potential and to support proper growth and nutrition in rats.[14] The choice of a 15% T. occidentalis dietary incorporation was reported by earlier studies showing that beyond 15% incorporation in the diet, the seeds resulted in significant hyperglycemia and lipid abnormalities.[15] Since medications should be available in quantities that are large enough to reverse a disorder or inhibit its onset, without resulting in adverse pathological conditions or undesirable side effects, the 15% level of incorporation was chosen for this study.
Exogenous estrogen administration resulted in a case of biochemical castration in rats in the test group and control I group. Intact rats remain sensitive to inhibition of pituitary function by estrogen.[16] The Leydig cells of the testes are known to produce testosterone as a result of stimulation by leutenizing hormone, a pituitary hormone. Dormancy of the testes results from a sustained inhibition of pituitary function, such that the testes atrophy. The production of biochemical castration is a known indirect effect of estrogen.[17,18] Administration of estradiol valerate in combination with DHT, therefore, led to biochemical castration in the rats and mimicked the situation observed in andropause, while maintaing serum concentrations of the reduced equivalent of testosterone, DHT, so as to sustain basic physiologic processes that require androgens, like the maintenance of heart beat.
The finding that the test diet was able to inhibit, albeit modestly, the biochemical castration as induced by exogenous estrogen administration, as seen in the modest increase in testes weights and relative testes weights of rats in the test group, relative to control I, is noteworthy. Apparently, certain phytochemical agents present in the seeds are able to interfere with estrogen inhibition of pituitary action or directly encourage tissue growth and differentiation in the testes. It is known that the regulation of testosterone production could be at the level of leutenizing hormone, cyclic adenosine monophosphate (cAMP), or any of the numerous mitochondrial and cytosolic proteins believed to play a role therein.[19] The suspected interference by agents in the seeds could be at any of these points and warrants further investigation.
The physiologic state of the testes can be assessed by their secretory capacity which in turn can be measured by determining the concentration of testosterone in serum. This follows from the premise that the Leydig cells of the testes have the primary function of biosynthesizing testosterone.[19] In fact it is known that any reduction in the concentration of androgens, androgen receptor expression, and androgen signaling results in decreased secretory activity of the testes and in apoptosis in the testicular epithelium.[20] For these reasons, the serum testosterone concentrations were measured as a surrogate assessment for testicular secretory function.
Interestingly, the test diet resulted in a higher concentration of testosterone in the serum of the test rats, relative to control I. This may be as a result of increased synthesis of testosterone due to an increased testicular mass or due to a reversal in the estrogen-induced negative feedback on the pituitary. The testosterone relative to testes weight index depicts the quantity of testosterone produced per gram of testicular tissue. The index shows that the testes of the rats that received exogenous hormones produced more testosterone per gram of tisssue, relative to their counterparts that did not receive the hormones. The test diet apparently caused an increase in this index for the test group such that its value was significantly higher than those of the three control groups. The increased concentration of testosterone produced per gram of testicular tissue may be due to a selective preservation of Leydig cells during testicular atrophy or as a result of increased biosynthetic activity of the testes under physiologic stress. It is also possible that the increased concentration of DHT (in the hormone treated rats) spared (by inhibition) the conversion of testosterone to DHT by the enzyme steroid 5α-reductase, such that testosterone concentrations in the test group and control I were higher than those of their counterparts that did not receive the hormones. Though these are speculations, it is important to note that the test diet ensured an increased synthesis of testosterone per gram of testicular tissue in the test group, relative to (more importantly) the control I group.
Since andropause results in a drop in serum testosterone levels,[21] any treatment that improves the testosterone concentration directly or indirectly (by increasing hypothalamic–pituitary responsiveness, testicular mass, and secretory activity) would be useful in ameliorating the negative effects of the debilitating disorder. This is the major merit of this study. Though the diet was not able to restore the testes weights and serum testosterone concentrations of rats in the test group to levels found in the control groups that did not receive the hormones, it is important to realize that any alleviation of the symptoms of andropause (no matter how small it is) is beneficial to the sufferer. The supranormal doses of estradiol administered to the rats was neccesary to achieve biochemical castration and the induction of andropause within a short time (experimental feasibility), but such levels may be difficult to achieve in aging men. This may necessarily imply that the results could be better if lower estradiol levels were at play.
It is important to note that there are as much inter-species differences as there are similarities in nature, such that an outright extrapolation of these findings to the human population, while enticing, is discouraged, pending the outcome of further studies. This work is limited by the administration of the hormones at high doses, for a short duration (28 days) instead of a gradual prolonged administration of lower doses of the agents. The physiologic stress (on the rats) and ethical concerns inherent in injecting rats on alternate days beyond 28 days due to the unavailability of subcutaneous hormone-bearing stents, however, warranted and justifies the method we used.
In conclusion, a 15% dietary incorporation of the seeds of T. occidentalis Hook f. modestly inhibitted the induction of experimental andropause (indicated by biochemical castration) by exogenous hormone administration. The seeds may, therefore, be useful to aging men in the prevention of andropause.
Source of Support
Nil.
Conflict of Interest
None declared.
References
- Biundo B. Treating andropause: Prohormones and hormone metabolic modifiers. Int J Pharm Compound 2001;5:351-3.
- Tan RS. Androgen: Introducing the concept of “relative hypogonadism” in aging males. Int J Impot Res 2002;14:319.
- Morley JE. Hormones and the aging process. J Am Geriatr Soc 2003;51: S333-7.
- Stellato RK, Feldman HA, Hamdy O, Horton ES, McKinlay JB. Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: Prospective results from the massuchusetts male aging study. Diabetes Care 2000;23:490-4.
- Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev 2005;26:833-76.
- Harman S. Testosterone in older men after the institute of medicine report: Where do we go from here? Climacteric 2005;8:124-35.
- O’Donnell AB, Araujo AB, McKinlay JB. The health of normally aging men: The massachusetts male aging study (1987-2004). Exp Gerontol 2004;39:975-84.
- Matsumoto AM. Andropause: Clinical implications of the decline in serum testosterone levels with aging in men. J Gerontol Med Sci 2002;57A: M76-99.
- Tariq SH, Haren MT, Jim MJ, Morley JE. Andropause: Is the emperor wearing any clothes? Rev Endocr Metab Disord 2005;6:77-84.
- Hayflick L. The future of aging. Nature 2000;408:267-9.
- Travison TG, Araujo AB, Kupelian V, O’Donnell AB, McKinlay JB. The relative contributions of aging, health and lifestyle factors to serum testosterone decline in men. J Clin Endocrinol Metab 2007;92:549-55.
- Nieschlag E, Behre HM, Gooren LJ, Kaufman JM, Legros JJ, Lunenfeld B, et al. Investigation, treatment and monitoring of late-onset hypogonadism in males: ISA, ISSAM and EAU recommendations. J Androl 2006;27:135-7.
- Turkes A, Turkes AO, Joyce BG, Read GF, Riad-Fahmy D. A sensitive solid phase enzyme immunoassay for testosterone in plasma and saliva. Steroids 1979;33:347-59.
- Ejike CE, Ugboaja PC, Ezeanyika LU. Dietary incorporation of boiled fluted pumpkin (Telfairia occidentalis Hook F) seeds: 1. Growth and toxicity in rats. Res J Biol Sci 2010;5:140-5.
- Ejike CE, Udensi AH, Ezeanyika LU. Dietary incorporation of boiled fluted pumpkin (Telfairia occidentalis Hook f) seeds: 2. Alterations in blood glucose concentration and serum lipid profile of rats. Res J Biol Sci 2010;5:146-9.
- McPherson SJ, Ellem SJ, Simpson ER, Patcher V, Fritzemeier K, Risbridger GP. Essential role of estrogen receptor β in stromalepithelial regulation of prostatic hyperplasia. Endocrinology 2007;148:566-74.
- Bianco JJ, Handelsman DJ, Pederson JS, Risbridger GP. Direct response of the murine prostate gland and seminal vessicles to estradiol. Endocrinology 2002;143:4922-33.
- Gooren LJ, Toorians AW. Significance of oestrogens in male (patho)physiology. Ann Endocrinol 2003;64:126-35.
- Payne AH, Youngblood GL. Regulation of expression of steriodogenic enzymes in leydig cells. Biol Reprod 1995;52:217-25.
- Wright AS, Thomas LN, Douglas RC. Relative potency of testosterone and DHT in preventing atrophy and apoptosis in the prostate of the castrated rat. J Clin Invest 1996;98:2258-63.
- Orwoll E, Lambert LC, Marshall LM, Phipps K, Blank J, Barrett-Connor E, et al. Testosterone and estradiol among older men. J Clin Endocrinol Metab 2006;91:1336-44.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.