Biochemical Markers of Oxidative Stress in Patients with Inflammatory Bowel Diseases
2 Department of Biochemistry, Ludwik Rydygier Collegium Medicum in Bydgoszcz, Nicolaus Copernicus University, Toruń, Poland
3 Department of Biochemistry, Institute of Physical Education, Kazimierz Wielki University in Bydgoszcz, Poland, Email: augustynska@op.pl
Received: 30-Apr-2022, Manuscript No. AMHSR-22-62353; Editor assigned: 04-May-2022, Pre QC No. AMHSR-22-62353(PQ); Reviewed: 18-May-2022 QC No. AMHSR-22-62353; Revised: 01-Jul-2022, Manuscript No. AMHSR-22-62353(R); Published: 07-Jul-2022
Citation: Augustynska B, et al. Biochemical Markers of Oxidative Stress in Patients with Inflammatory Bowel Diseases. Ann Med Health Sci Res. 2022;12:1-7.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: Inflammatory Bowel Diseases (IBD) is a group of diseases of unexplained etiology, characterized by periods of remissions and exacerbations. Reactive Oxygen Species (ROS) as far as disorders of balance between levels of prooxidants and antioxidants may also participate in upraise of IBD. The aim of our study was an assessment of the antioxidative barrier of the organism in patients with inflammatory bowel disease.
Material and methods: The study group consisted of 99 patients (80 with IBD as a study group and 19 healthy as a control group) of Jan Biziel University Hospital in Bydgoszcz, Poland. Venous blood was a material for biochemical analysis: HT, GSH, GPXp, GPXRBC, GST, GR, SOD-1, MDA, NO2-/NO3- and CP. Results: There were statistically significant differences in oxidative stress parameters observed between study group and control group, especially concerning HT, GSH, GPXRBC, GST, SOD-1, MDA and NO2-/NO3.
Discussion: The assumption that increased activity of antioxidative compounds may constitute a defense against the influence of oxidative stress may be true. Their decreased activity may participate in lowering organism abilities to defend against oxidative stress and cause the development of free radical diseases. Further studies as far as targeted preventive strategies are needed.
Keywords
Oxidative stress; Anti oxidative barrier; Inflammatory bowel disease
Abbreviations
CAT: Catalase; CP: Oxidase Ceruloplasmin; GPX: Glutathione Peroxidase; GPXp: Glutathione Peroxidase (plasma); GPXRBC: Glutathione Peroxidase (Red Blood Cells); GR: Glutathione Reductase; GSH: Glutathione; GST: Glutathione S-Transferase; H2O2: Hydrogen Peroxide; HT: Hematocrit; IBD: Inflammatory Bowel Diseases; MDA: Malondialdehyde; NO2: Nitrate; NO3: Nitrite; O2: Singlet Oxygen; O2: Superoxide Anion; O3: Ozone; OH: Hydroxyl Radical; RO: Alkoxy Radical; ROO: Radical Peroxide; ROS: Reactive Oxygen Species; SD: Standard Deviation; SOD-1: Superoxide Dismutase.
Introduction
Inflammatory Bowel Diseases (IBD) is a group of diseases of unexplained etiology, characterized by periods of remissions and exacerbations. Two main diseases characterized by diverse etiopathology belong to the group above: Ulcerative Colitis (CU) and Crohn Disease (CD). Main causes are genetical, improper diet, improper reaction of the immune system to enteric bacteria or drugs [1].
Reactive Oxygen Species (ROS) as far as disorders of balance between levels of prooxidants and antioxidants may also be a cause of IBD. Prevalence of the oxidative reaction causes the development of processes described as oxidative stress [2]. The result of the reaction above is a change in the protein structure and function, oxidation of cell membranes and DNA damages [3]. The result of oxidative stress is lipid peroxidation [4]. It is a process of oxidation of the unsaturated fatty acids, constituting elements of phospholipids. Lipids peroxidation causes the emergence of their peroxides. The main product of lipids peroxidation is Malondialdehyde (MDA).
MDA concentration increases if production ROS increases, thus cell membrane permeability changes [5]. Colonization of large intestine is caused by the huge amount of bacteria stimulating the GALT (Gut Associated Lymphoid Tissue) system. The main task of GALT system is discrimination between symbionts and pathologic bacteria. Normal conditions are described as a balance between intestinal microflora and GALT functioning. Normal functioning of GALT depends on tightness of the intercellular junctions and the mucus layer. Hydrogen peroxide, produced by colonocytes, contributes to intercellular junction damages [6]. It causes permeation of bacteria to lamina propria, production of proinflammatory cytokines and stimulation of T cells [7].
Increased amount of T cells, B lymphocytes, monocytes, mast cells and macrophages within intestinal mucosa is observed in the course of IBD. The number of Poly Morpho Nuclear leukocytes (PMN) in lamina propria also increases. Especially neutrophiles, releasing significant amounts of proteases, contribute to intercellular junction unsealing. It leads to accumulation of the aforementioned cells and penetrating the intestinal mucosa. PNMs, T cells, and B lymphocytes produce ROS (such as superoxide anion, hydroxyl radical, etc.) to destroy bacteria [8].
A number of enzymes take part in the process of ROS production. Super Oxide Dismutase (SOD-1) in the reaction of dismutation of superoxide anion leads to the production of hydrogen peroxide, which is next decomposed through catalase [9]. Glutathione Pero Xidase (GPX) takes part in the neutralization of hydrogen peroxide, but GPX need reduced glutathione (GSH) for action [10].
GSH as cosubstrate takes part in reactions of hydrogen peroxides and lipid peroxides [11]. Forementioned reactions result in the production of glutathione disulfide (GSSG), reduced by Nicotinamide Adenine Dinucleotide Phosphate (NADPH) during reaction catalyzed by Glutathione Reductase (GR). The main role of GR is keeping reduced sulfhydryl groups of proteins with the participation of glutathione transhydrogenase. GR also participates in the removal of noxious substances from cells (xenobiotics and their metabolites, products of lipids peroxidation, etc.) through enzymatic reactions or catalyzed by glutathione S-transferase [4].
Also worthy of note is Cerulo Plasmin (CP)–copper binding protein. CP contributes to decreasing of copper concentration in serum and prevents participating of copper in free-radical reactions [12]. Inflammatory bowel disease constitutes a group of diseases of barely known etiology. One of the possible causes may constitute free radical reactions increasing oxidative stress. Thus we try to describe antioxidant abilities of the organism in the course of the aforementioned diseases. The aim of our study was an assessment of the antioxidative barrier of the organism in patients with inflammatory bowel disease.
Materials and Methods
Materials
The study group consisted of 80 patients of clinic of Jan Biziel University hospital in Bydgoszcz, Poland. Study group consisted of 80 patients with IBD (mean age 32, 66 ± 17, 4 years). Reference group consisted of 19 healthy people mean age 22.24 ± 3.64 years, with no health conditions (fever, contagious diseases, blood clots, pregnancy, kidney and liver conditions, cancer, inflammations and hypertension were excluded as a contraindication during general medical examination). Every person was fasted till the last blood sample collection. Study was conducted in accordance with the declaration of helsinki and the guidelines for Good Clinical Practice (GCP). Freely given written informed consent was obtained from every participant before the study. Approval was granted by a Ludwik Rydygier Collegium Medicum in Bydgoszcz, Nicolaus Copernicus University, Toruń, Poland ethics review board KB 551/2013
MethodsThe material for biochemical analysis was venous blood collected in an amount of approx. 8 ml of the antecubital vein into lithium heparin tubes and tubes without anticoagulant. Blood samples were collected at 8.00 A.M. Then, collected material was transported to the department of biochemistry of Nicolaus Copernicus University Collegium Medicum in Bydgoszcz. Tests were carried out on the same day, within approximately 1 hour of material collection. In the experiment, activity of following biochemical markers of oxidative homeostasis was assessed: oxidase Ceruloplasmin (CP), Glutathione (GSH), Glutathione Reductase (GR) Malondialdehyde (MDA), Glutathione Peroxidase (GPX), glutathione S-transferase (GST) and superoxide dismutase (SOD-1). He was assayed using impeller-based method. From the blood drawn into tubes without anticoagulant (approximately 3 ml) serum was obtained by centrifugation of the material over 5 minutes at 5000 × g, and then it was transferred to Eppendorf tubes and frozen at -80°C. The prepared serum was stored to determine the activity of the oxidase Ceruloplasmin (Cp). Before preparing the hemolysate, 500 μl bloods was collected to determine the levels of GSH in the erythrocytes. The remaining aliquot of blood (approx. 5 ml) was centrifuged to obtain plasma, wherein the concentration of nitrate/nitrite (NO2-/NO3-) was determined. The remaining cells were used for the preparation of the hemolysate, wherein the dialdehyde malonic concentration (MDA) and the activity of the enzymes: Glutathione Peroxidase (GPX), Glutathione S-Transferase (GST), Gluatathion Reductase (GR) and Superoxide Dismutase (SOD-1) were determined. The concentration of GSH was assayed using the Beutler method [13]. The principle of this method is based on the reaction of reduction of the disulfide compound Dithio-Bis-2-Nitrobenzoic acid (DTNB) by compounds containing sulfhydryl groups. In blood free sulfhydryl groups unrelated to proteins derived almost only from GSH. The product of the described reaction is a compound of yellow color. Color density was measured at wave length 412 nm. In the calculations the molar absorption coefficient was used, which, when attached to the mentioned wavelength, is equal to 13.6 (mol-1 x l x cm-1). The results were expressed in mmol/LRBC. The coefficient of variation for this method was 2.4%. The activity of GR in erythrocytes was assayed by a spectrophotometric measurement of NADP formation rate. NADP is the result of the reduction of glutathione oxidase in a reaction catalyzed by glutathione [14]. Change of absorbance was measured at wavelength 340 nm, and the result was expressed in U/g Hb. The variation coefficient for this method is 3.8%. The activity of GPX in erythrocytes was assayed by a two-stage Paglia and Valentine method [15]. In the first stage, GPX reacts with tert-butyl peroxide and reduced glutathione (GSH). The product of this reaction is glutathione disulfide (GSSG). The second stage involves the action of Glutathione Reductase (GR) reducing GSSG to GSH with the participation of NADPH+H+ as a regulator. NADPH oxidation results in a reduction in absorbance at a wavelength of 340 nm, which is measured spectrophotometrically. Activity of GPX was calculated based on the loss of the reduced form of coenzyme in time (Wartburg test). In the calculations, millimolar absorption coefficient for NADPH at wave length 340 nm, equal to 6.22 (mmol-1 x l x cm-1) was used. The results were expressed in U/g Hb, where 1 μmola oxidation of NADPH in one minute at T=25°C was adopted as a unit of enzyme activity. The coefficient of variation for this method was 2.9%. Determination of GST activity in erythrocytes was performed according to the Habig method [15]. In this method, there is a decrease in absorbance (which is measured at wavelength of 340 nm) due to the formation of a conjugate of glutathione (GSH) with 1-chloro-2, 4-dinitrobenzene (CDNB). The decrease in absorbance is proportional to the glutathione Stransferase activity. GST activity assay was carried out in the presence of phosphate buffer and CDNB. The results were expressed in nmol/CDNB-GSH/mg Hb/min. SOD-1 activity in erythrocytes was determined using the Misra and Fridovich method, which is based on the inhibition of adrenaline oxidation reaction by superoxide dismutase at pH 10.2 [8]. The increase in absorbance was measured at wavelength of 480 nm. It is proportional to the increase in the concentration of oxidation products of adrenaline. The activity of SOD-1 was expressed in U/g Hb. The amount of enzyme which inhibits the oxidation of adrenaline 50% was adopted as a U unit. The coefficient of variation for this method is 6.3%. The concentration of MDA in the erythrocytes was determined by Placer, et al. method, which is based on the reaction of a thiobarbituric acid and certain products of lipid peroxidation, mainly MDA, in an acidic environment and at elevated temperature [16]. This reaction produces a colored product, the color intensity of which was measured at wavelength of 532 nm. In the calculations, the millimolar absorption coefficient of 156 (mmol-1 x l x cm-1) was used. The result was expressed in mmol/g Hb. The coefficient of variation for this method was 3.5%. The concentration of nitric oxide was determined using the indirect method according to Marlett, determining the concentration of nitrate/nitrite in plasma. The method is based on the reaction between the nitrate anion and anion from N-(1-naphthyl) ethylenediamine, in the sulfanilic acid environment (Griess reaction) [17]. This reaction produces a colored complex whose absorbance is measured at a wavelength of 545 nm. It is directly proportional to the concentration of nitrates and nitrites in the studied sample. The result was expressed μml/L. Ceruloplasmin oxidase activity was determined using the method of Ravin [18]. The principle of the method is based on oxidation of substrate PPhenyl Diamine (PPD) by ceruloplasmin at a final purplecolored product. Absorbance measurement was made at wavelength of 530 nm. This product is so called ‘the principle of Bandrowski’ (product formed from three molecules of the substrate). Results were expressed in international units.
Statistical analysis
Statistical analysis was made using software SPSS. Where available, measured data was described as mean with Standard Deviation (SD) or median with minima and maximal values. Normality of distribution was checked using Schapiro-Wilk test. According to the needs, for data sets with normal distribution t-test was applied, and for the other data sets U-Mann Whitney test was applied. P value was set at 0.05.
Results
There were statistically significant differences in oxidative stress parameters observed between study group and control group (Table 1).
| Table 1: Differences in oxidative stress parameters observed between study group and control group. | |||||
|---|---|---|---|---|---|
| Parameter (%) | Study group (n=80) | Reference group (n=19) | p value | ||
| Mean | SD | Mean | SD | ||
| HT | 40.15 | 4.206 | 43.34 | 3.253 | 0.003 |
| GSH (mmol) | 2.793 | 0.3730 | 2.208 | 0.1625 | ≤ 0.01 |
| GPXp (U) | 184.480 | 243.9192 | 246.047 | 48.2888 | n.s. |
| GPXRBC (U) | 15.336 | 2.2086 | 18.911 | 2.2573 | ≤ 0.01 |
| GST (nmol) | 2.872 | 0.6929 | 2.495 | 0.4236 | 0.025 |
| GR (U) | 56.278 | 9.4105 | 55.147 | 11.2139 | n.s. |
| SOD-1 (U) | 2471.19 | 148.555 | 2805.26 | 184.771 | ≤ 0.01 |
| MDA (mmol) | 0.28362 | 0,024803 | 0.25289 | 0.033637 | ≤ 0.01 |
| NO2-/NO3- (µmol/L) | 1.4064 | 1.24503 | 0.8121 | 0.63482 | 0.047 |
| CP (IU) | 1206.121 | 415.9030 | 1340.358 | 542.4489 | n’s. |
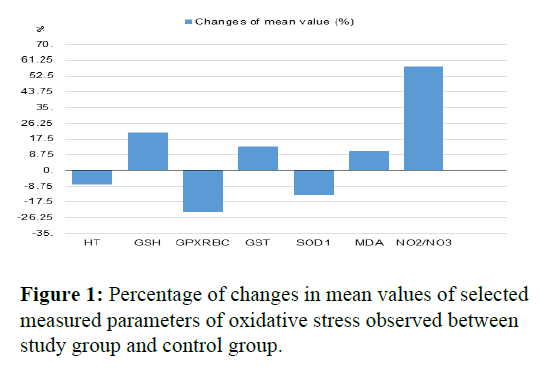
Mean concentration of the reduced glutathione in erythrocytes was higher in the study group compared with the control group (p ≤ 0.01). The activity of glutathione peroxidase was lower in the study group compared with the control group (p ≤ 0.01). The activity of this enzyme in erythrocytes was higher in the study group compared with the control group (p ≤ 0.01). There was also higher activity of the glutathione-S-transferase observed in the study group compared with the control group (p=0.025). The activity of SOD-1 was significantly lower in the study group compared with the control group (p ≤ 0.01). The concentration of MDA was significantly higher in the study group compared with the control group (p ≤ 0.01). The concentration of nitric oxide in plasma (measured indirectly based on the concentration of nitrates/nitirtes) was significantly higher in the study group compared with the control group (p=0.047). Values of Ht was significantly lower in the study group compared with the control group (p=0.003). Other statistically significant differences between the study and the control group were not observed. In the study group compared with the control group none changes in the levels/ concentration of the measured parameters of the oxidative stress were observed in GPXp, GR and CP. Contrary, it was shown that in the study group compared with the control group there was significant decrease about 23,31 and 13,52% in the level concentration of GPXRBC and SOD respectively. The study group demonstrated significant increase of 20,95%, 13,13%, 10,83% and in the level/concentration of GSH, GST, MDA and NO2-/NO3- respectively (Figure 1).
Discussion
Development of diseases based on free radicals may take place when the intensity of the oxidative stress exceeds adaptability of the organism. Prevention of results of the extensive ROS production elements of the antioxidative barrier of the organism should constitute integrated and efficient system allowing for control of free radicals production and defend against their biological effects. Such system consists of both, the enzymatic and non-enzymatic mechanisms making ROS harmless. IBD constitute a group of diseases characterized by various and not fully known etiology. One of the possible causes of their uprise may be free radical reactions and disorder of balance between levels of prooxidants and antioxidants. Drugs used to treat aforementioned diseases show both anti-inflammatory and antioxidant action, what is important taking into consideration free radical causes of IBD [19]. The assumption that increased activity of antioxidative compounds may constitute a defense against the influence of oxidative stress may be true. Their decreased activity may participate in lowering organism abilities to defend against oxidative stress and cause the development of free radical diseases. The main limitation of the previous studies constitutes a relatively low number of coherent results explaining associated problems in comprehensive way. Our study constitutes another step toward better understanding free radical and antioxidative processes in patients with IBD. ROS are produced in many biological processes. Released in physiological amounts, they play the role of mediators and regulators providing normal functioning of cells [20]. ROS influence on cells depends on their concentration and duration of action, and their production should be under strict control of both, the enzymatic antioxidant system and no enzymatic antioxidant system. When such defense system is inefficient and production of ROS increases there may be disturbed the balance between provident and antioxidant processes within the cell. Aforementioned phenomenon is called oxidative stress. Its consequences are damages of cell elements or even disorders of cells and tissues function [21]. Mechanism of ROS production is cascading. During the first stage of reaction, electron is joined to the oxygen producing superoxide radical anion [22]. Superoxide radical anion is a basis for the other ROSs. SOD participates in the decomposition of superoxide radical anion into oxygen and hydrogen peroxide [23]. Showed decreased activity of this enzyme in neutrophils from tissue cultures in patients with IDB compared to healthy people [18]. Showed higher activity of this enzyme in erythrocytes of patients with UC compared with the reference group. Own studies showed a decrease in activity of SOD-1 in erythrocytes of patients with IBD compared with control group. Decreased activity of SOD-1 may participate in the accumulation of superoxide radical anion. Its high level may also inhibit the activity of GPX. Own studies confirm decrease of GPX activity in erythrocytes of patients with IBD compared to control group. Similar outcomes were observed [24]. There was observed decreased activity of this enzyme in erythrocytes of people with IBD treated due to anemia. From the other side [25]. Showed lack of the statistically relevant differences in GPX activity in serum of patients with IBD compared to healthy people. The results of own study were similar: statistically relevant differences in GPX activity in serum of patients with IBD compared to healthy people have been not observed. Anaemia belongs to systemic complications of IBD. It constitutes a cause of quality of life decrease and hospitalization [7]. Haematological diagnostics of IBD consists of measurements of Hemoglobin (Hb) concentration, red blood cells count, white blood cells count, Hematocrit (Ht) and erythrocyte sedimentation rate. Our results for Ht were significantly lower in the study group compared to the reference group. GSH consists the main element of defense against oxidative stress. Observed decreased GSH level in patients with IBD compared to control group. Showed decreased level of GSH in patients with CU compared with the reference group [26]. Did not observe statistically relevant differences in GR level in tissues of patients with chronic CU and CD, but they observed that GSH concentration is increased during the remission of CU compared to active period of the disease. Own results showed an increase in the GSH level in IBD patients compared with control group. Glutathione is one of the most important elements of the antioxidative cell defense system due to its ability to reduce the peroxides and keep normal levels of SH groups within proteins [27]. Moreover, it plays the role of the intracellular high capacity redox buffer and ROS destroyer. GST activity also helps to assess disorders of the antioxidant barrier in erythrocytes. The biologic action of this enzyme is based on participation in the second phase of detoxication of xenobiotic. GST may also participate in regulation of GPX activity [28]. Own studies showed increased activity of this enzyme in the study group compared to control group. GR plays adjuvant function supporting main elements of the antioxidative protective barrier. It reduces oxidative glutathione and brings back its antioxidative abilities, what allow for its reuse in reactions catalyzed by GPX and GST [29]. Own studies showed lack of statistically relevant differences in GR activity between study group and the reference group. Results of damages caused by the oxidative stress can be measured indirectly by the concentration of the thiobarbituric acid [30]. MDA is the most commonly used. MDA is a result of lipids peroxidation and its concentration increases in the case of increased ROS production in the body. It causes a change in the membrane cell permeability, disturbances of oxidative phosphorylation within mitochondria, and as a consequence induction of apoptosis. Statistically relevant increase of MDA concentration constitutes a useful indicator of the disorders of antioxidative barrier of the body [31]. Showed statistically relevant increase of MDA concentration in serum of patients with Crohn’s disease compared with the reference group. Despite MDA concentration in patients with CU was also significantly higher than in healthy people, aforementioned data were not statistically relevant and need for further research. Own studies showed increased MDA concentration in study group compared with control group. From the other side [32]. Showed a lack of differences in MDA concentration between IBD patients and healthy people. Nitric Oxide (NO) is also a nonenzymatic antioxidative factor. It plays significant role in the regulation of the cell membrane tension, ROS capturing and protection against lipids peroxidation [33]. The role of NO is twofold: is antioxidant and participates in ROS production [34]. Our own study consisted of measuring nitrites to nitrates ratio, what allow for assessment of the NO metabolism. Statistically relevant increase of the NO metabolism in the blood was observed in the study group compared to the reference group. Similar results were showed by [35]. Increase of NO level was observed in patients with IBD compared with control group. The increase of the concentration of NO in patients with IBD is a result of increased activity of NO synthesis in this group of diseases. Abilities to defend against ROS also depend on the action of endogenous proteins, which have antioxidant properties. CP belongs to such proteins it binds transition metal ions what decreases free radical reactions. No statistically relevant differences concerning concentration of this protein between study group and reference group were observed. Showed that overexpression of CP might be engaged in uprise and development of the Crohn’s disease.
Conclusion
IBD has a complex and still largely unknown etiopathology. One of the possible causes can be traced to free radical reactions and precisely to disorders in the equilibrium between prooxidants and antioksidants. On the basis of obtained results, it seems that prooxidative factirs play an essential role in pathogenesis of IBD. Main directions of further study are twofold: confirmation of current outcomes during randomized controlled trials on a bigger group of patients (including various etiologies) and join them and build comprehensive, evidence-based theory in the area of upraise and development of the diseases based on free radicals.
Author’s Contribution
All authors read and approved the final manuscript. G.M. and B.A. conceived and designed the project. G.M, B.A. D.K. collected the data. G.M, B.A, M.K. analyzed and interpreted the data. B.A. drafted the manuscript. All authors read and approved the final manuscript.
Conflict of Interests
The authors declare that there is no conflict of interests in this study.
References
- Weiss G, Gasche C. Pathogenesis and treatment of anemia in Inflammatory bowel disease. Haematologica. 2010; 95:175.
- Bokov A,Chaudhuri A. The role of oxidative damage and stress in aging. Mech of Aging Dev. 2004;125:811-826.
- Vertuani S, Angusti A, Manfredini S. The antioxidants and proantioxidants network: an overview. Current pharmaceutical design. 2004;10:1677-1694.
- Rahman T, Hosen I, Islam MMT, Shekhar HU. Oxidative stress and human health. Adv Biosci Biotechnol. 2012;3:997–1019.
- Ray PD, Huang BW, Tsuji Y. Reactive Oxygen Species (ROS) homeostasis and redox regulation in cellular signaling. Cellular signaling.2012;24:981-90.
- Pravda J. Radical induction theory of ulcerative colitis. World J Gastroenterol. 2005;11:2371.
- Theurl I, Aigner E, Theurl M. Manfred N, Markus S, Andrea S, et al. Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: diagnostic and therapeutic implications. Blood. 2009;113:5277:86.
- Pavlick KP, Laroux FS, Fuseler J, Jonathan MD, David YK, Matthew BG, et al. Role of reactive metabolites of oxygen and nitrogen in inflammatory bowel disease. Free Rad Biol Med. 2002;33:311-322.
- Verspaget HW, Peña AS, Weterman, CB Lamers, Gordon LT, Mary FO, et al. Diminished neutrophil function in Crohn’s disease and ulcerative colitis identified by decreased oxidative metabolism and low superoxide dismutase content. Gut.1998;29:223 228.
- Ruan EA, Rao S, Burdick JS. Steven J. Stryker Glutathione levels in chronic inflammatory disorders of the human colon. Nutr Res. 1997;17:463-73.
- Hernanz A, Fernandez-Vivanocos E, Montiel C, J Vazquez, F Arnalich. Changes in the intracellular homocysteine and glutathione content associated with aging. Life Sci. 2000;67:1317-1324.
- Bunker VW. Free radicals, antioxidant and ageing. Med Lab Sci;1992;49:299-312.
- Beutler E. Red cell metabolism. [In:] Beutler E. A manual of biochemical methods. grune-stratton new york.1975:11-12.
- Flohé L, Günzler WW. Assays of glutathione peroxidase. Methods in Enzymol. 1984;105:114-121.
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med.1967; 70:158-169.
- Placer ZA, Cushman LL, Johnson BC. Estimation of product of lipid peroxidation malondialdehyde in biochemical systems. Anals Biochem. 1966; 16:359-64.
- Griess P. Bemerkungen zu der Abhandlung der HH. Weselky und Benedikt ueber einege Azoverbindungen. Chem Ber.1879;12:426-428.
- Rana VS, Sharma S, Prasad KK. Role of oxidative stress & antioxidant defence in ulcerative colitis patients form north India. Indian J Med Res. 2014; 139:568.
- Mulder TPJ, Verspaget HW, Janssens AR, Bruin PA, Peña AS, Lamers CB,et al. Decrease in two intestinal copper/zinc containing proteins with antioxidant function in inflammatory bowel disease. Gut.1991;32:1146-1150.
- Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47-95.
- Sido B, Hack V, Hochlehnert A. Impairment of intestinal glutathione synthesis in patients with inflammatory bowel disease. Gut. 1998;42:485-492.
- Stadtman ER, Levine RL. Protein oxidation. Annals of the New York Academy of Sciences. 2000;899:191-208.
- Vaiopoulou A, Gazouli M, Papadopoulou A. Serum protein profiling of adults and children with Crohn disease. J Pediatr Gastroenterol Nutr. 2015; 60:42.
- Krzystek-Korpacka M, Neubauer K, Berdowska I, B Zielinski, L Paradowski, A Gamian, et al. Impaired erythrocyte antioxidant defense in active inflammatory bowel disease: impact of anemia and treatment. Inflamm Bowel Dis. 2010;16:1467-4175.
- Akman T, Akarsu M, Akpinar H, Resmi H, Sezer E. Erythrocyte deformability and oxidative stress in inflammatory bowel disease. Dig Dis Sci. 2012;57:458-464.
- Ravin HA. Improved colorimetric enzymatic ceruloplasmin assay. J Lab Clin Med. 1961;58:161-168.
- Itoh K, Ishii T, Wakabayashi N, et al. Regulatory mechanism of cellular response to oxidative stress. Free Radic Biol Med. 1999;31:319-324.
- De Sousa CV, Sales MM, Rosa TS, Lewis JE, de Andrade RV, Simões HG. The antioxidant effect of exercise: A systematic review and meta-analysis Sports Med. 2017;47:277-293.
- Janero DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Rad Biol Med. 1990;9:515-40.
- Alzoghaibi MA, Al Mofleh IA, Al-Jebreen AM. Lipid peroxides in patients with inflammatory bowel disease. Saudi J Gastroenterol. 2007;13:187.
- Trougakos IP, Gonos ES. Regulation of clusterien/apolipoprotein J, a functional homologue to the small heat shock proteins, by oxidative stress in aging and age-related diseases. Free Radic Res. 2006;40:1324-34.
- O’Donnell VB, Chumley PH, Hogg N, Bloodsworth A, Darley-Usmarv VM, Freeman BA, et al. Nitric oxide inhibition of lipid peroxidation: kinetics of reaction with lipid peroxyl radicals and comparison with alphatocopherol. Biochemistry. 1997;36:15216-1523.
- Opara EC. Oxidative stress. Dis Mon. 2006;52:183-198.
- Avdagić N, Zaćiragic A, Babic N, et al. Nitric oxide as a potential biomarker in inflammatory bowel disease. Bosn J Basic Med Sci. 2013;13:1-5.
- Tüzün A, Erdil A, Inal V, Aydin A, Bağci S, Yeşilova Z, et al. Oxidative stress and antioxidant capacity in patients with inflammatory bowel disease. Clin Biochem. 2002;35:569-72.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.