Can Chronoscopic Reading in Whole Body Reaction Time be a Tool in Detecting Cognitive Dysfunction in Hypertensives? Findings from a Case Control Study
- *Corresponding Author:
- Dr. Vitthal Khode
Department of Physiology, SDM College of Medical Sciences, Sattur, Dharwad, Karnataka, India.
E-mail: drkhoday@yahoo.co.in
Abstract
Background: Hypertension on a long‑term basis can cause target organ damage, especially the central nervous system, which can affect cognition. It is known that difference between simple and choice reaction time (RT) implies time required for cognition. Although delayed RTs indicate involvement of cognition, they cannot quantify how much time is required for cognition. Aim: Recording chronoscpic RT to quantify time required for cognition in hypertensives and compare them with controls. Subjects and Methods: This is a hospital‑based case–control study conducted (August 2010 to January 2011) on 118 subjects attending an outpatient department using visual and whole body reaction timers having criteria of age and hypertensive condition, compared with an equal number of age‑ and sex‑matched controls. Statistical analysis was carried out by Independent t test and duration of hypertension was correlated with whole body choice reaction time (WBCRT) C1 using Pearson’s correlation. Predictive value of WBCRT C1 was calculated by using the receiver operating characteristic curve. Results: The WBCRT C1 562.6 (108) ms was more delayed among hypertensives compared with controls 523.5( 98.8) ms. There was no significant correlation between duration of hypertension and WBCRT C1 (r = −0.064). The best cut‑off value for WBCRT C1when predicting cognitive dysfunction in hypertensive patients was 538.5 ms (sensitivity 76.2%, specificity 50%). Conclusions: WBCRT C1 can be a quantitative measurement of cognition. It can be used as a screening tool to detect cognitive dysfunction.
Keywords
Cognition, Hypertension, Visual reaction times
Introduction
Hypertension is a silent killer. On a long-term basis, it can cause target organ damage, especially the central nervous system. Hypertension is a risk factor for lowered cognitive performance.[1] In cross-sectional, prospective and longitudinal studies, inverse associations between blood pressure and cognitive performance level are observed over a wide range of systolic blood pressure (SBP), diastolic blood pressure (DBP) and mean arterial blood pressure (BP) levels.[2-6] Many hypertension-related changes in the brain have been identified and posited as the mechanisms underlying relations between BP and cognition.[1,2] Reviews of the literature indicate little in the way of consistent support.[7-10] Cross-sectional studies report either no interactions of age with BP or interactions in the opposite direction, such that SBP, DBP and mean arterial BP are more strongly related to cognition in younger than in middle-aged adults.[7-10] Reaction time (RT) is one of the reliable indicators of the time taken from onset of stimulus to appropriate response, which includes rate of processing of sensory stimuli by the central nervous system and its execution by motor response. It is known that a difference between simple and choice RT implies cognitive dysfunction.[11,12] Investigators have shown that choice RTs are delayed in hypertension. Although delayed RTs indicate involvement of central processing, they cannot quantify how much time is required for central processing.
In whole body choice reaction time (WBCRT), RT is split into two chronoscopic readings: WBCRT C1 and WBCRT C2. WBCRT C1 is the time required from visual stimuli to when the subject lifts his leg from the starting platform, which measures the time required for central processing or cognition. WBCRT C2 is the time required from visual stimuli to end task. WBCRT C2-C1 is the time required for peripheral response. The purpose of this study is to determine whether RTs, particularly WBCRT C1, can be a measure of cognitive dysfunction in hypertensives. The hypothesis of the present study was that WBCRT C1in hypertensives, without overt cerebrovascular disease, would be delayed. We determined visual reaction and whole body RTs, both simple and choice, and cognitive performance in normotensives and hypertensives without cerebrovascular disease, target organ damage or other vascular risk factors. We tried to determine the predictive value of WBCRT C1 in detecting cognitive dysfunction in them.
Subjects and Methods
Settings and participants
After getting approval of the Ethical Clearance Committee of the institution, this case–control study was carried out over 6 months (August 2010 to January 2011) with purposive sample, with the criteria of age and hypertensive condition. The selection of sample was carried out from the outpatient department (OPD) of medicine of our institution.
Design
One hundred and eighteen individuals participated in the study. The whole population was divided into two groups. Group 1 consisted of randomly selected known hypertensives more than 2 years of duration aged between 40 and 60 years who were attending the medical OPD. Group 2 consisted of randomly selected sex- and age-matched controls who attended the medical OPD for routine check-up. Sample size was determined by standard error obtained by a pilot study. Each individual was briefed about the study before start of study; its importance and procedural details and written consent of participants were taken before recording the various RTs. Following subjects were excluded from the study in both groups: Type 2 diabetics, smokers, retinopathy, motor neuron diseases, cardiovascular, cerebrovascular disorders, neuropathy and chronic renal disorders. We also excluded patients having chronic lower back pain or spasms, deformities of the spine, bones or joints (including advanced arthritis), spinal cord injuries or other damage to the nervous system, non-healing skin ulcers, current drug or alcohol dependence. Individuals taking any prescription medicine to prevent dizziness were also excluded.
Hypertension was diagnosed in patients who had blood pressure 140/90 mmHg or more or who were receiving antihypertensive medications. Medical history was obtained by self-report. Diabetics were excluded on the basis of the following criteria: Random blood sugar (RBS) >140 mg% or patients on antidiabetic therapy. The basic parameters and detailed history were recorded. General check-up of pulse, height, weight, food habits and exercise pattern were recorded. Random sugar levels were measured. Ophthalmic evaluation was done using the Snellen and Jeagers chart.
Equipment used for reaction timers
The reaction timers
• Visual reaction time
1. Visual simple reaction time (VSRT)
2. Visual choice reaction time (VCRT)
• Whole body reaction time
1. Whole body simple reaction time (WBSRT). (Chronoscope-1, Chronoscope-2 and Chronoscope 2-1).
2. Whole body choice reaction time (WBCRT). (Chronoscope-1, Chronoscope-2 and Chronoscope 2-1).
Anand Agencies, Pune, India, was the manufacturer of the research tool RT apparatus, with the chronoscope compartment showing time in milliseconds.
Procedure
After brief instructions, three trials for each of VSRT, VCRT, WBSRT and WBCRT were given and the individual RTs in milliseconds were recorded five times in both hypertensives and controls. An attempt was made to obtain at least five acceptable recordings for each participant. Measurements of the VSRT, VCRT and WBSRT were considered reproducible if the difference between the maximum and the minimum values did not exceed 50 ms. Reliability of the test was calculated based on the data obtained in a pilot study. Coefficient of correlation for VSRT was 0.927, with α error 0.9844. VSRT - The subject is instructed to press the right button as soon as the red light glows, and the chronoscope reading is recorded. VCRT - The subject is instructed to press the left button when the green light glows and the right button when the red light glows, and RT is recorded. WBSRT - The subject standing on the starting board is instructed to watch the glowing arrow and to step one leg on the stepping board in that single direction. Chronoscope-1 gives the time taken for lifting of the foot from the onset of the stimulus, whereas Chronoscope-2 gives the total time required for placing the foot on the stepping board from the onset of stimulus, and C2 - C1 gives the movement time from starting board to stepping board, which is the time taken for motor activity. WBCRT - The subject is asked to move either of the legs according to the direction of the glowing arrow, either to the right, front, left, behind and right again, which involves more cognition compared with WBSRT.
Statistical analysis
The results were tabulated separately and statistical results were presented as mean (SD). The software analyzer used was SPSS version 16 (2007, USA). The data were analyzed by independent t test, which indicates the level of difference between the groups, with significance at the 5% level using t Stat, i.e., P < 0.05. Pearson’s correlation was performed to find the correlation between duration of hypertension and WBCRT C1. To determine the accuracy and respective best cut-off values of WBCRT C1 for predicting cognitive dysfunction, the receiver operating characteristic (ROC) curves and their corresponding areas under the curve (AUC) were used. A P < 0.05 was considered statistically significant.
Results
As per Table 1, there was no significant difference in age. The mean ages of the controls and the hypertensives were 49.1 (6.5) and 50.7 (6.6) years, respectively. There were 36 males and 23 females in Group 1 and Group 2. Table 1 also shows the mean of the measured values of SBP, DBP, Body Mass Index (BMI) and RBS. There was a significant difference in SBP, DBP, BMI and RBS in hypertensives and controls (P < 0.001). There was no significant correlation between SBP, DBP and duration of hypertension with WBCRT C1 (r = 0.035, -0.109 and 0.229, respectively).
| Variables | Group 1 hypertension n=60 Mean (SD) |
Group 2 controls n=60 Mean (SD) |
t value | P value | |
|---|---|---|---|---|---|
| Age (years) | 50.7 | (6.6) | 49.1 (6.5) | −1.329 | 0.18 |
| Height (m) | 1.57 | (0.7) | 1.60 (0.8) | 2.050 | 0.04 |
| Weight (kg) | 66.2 (11.8) | 62.4 (11.5) | −1.767 | 0.08 | |
| BMI (kg/m2) | 26.69 (4.8) | 24.1 (3.4) | −3.586 | <0.01 | |
| SBP (mmHg) | 141.6 | (12.2) | 131.6 (6.2) | −5.322 | <0.01 |
| DBP (mmHg) | 87.4 (5.65) | 82.4 (5.3) | −4.912 | <0.01 | |
| RBS (mg%) | 110.8 | (19.9) | 97.4 (13.9) | −4.216 | <0.01 |
*P<0.05, BMI: Body mass index, SBP: Systolic blood pressure, DBP: Diastolic blood pressure, RBS: Random blood sugar
Table 1: Demographic variables of hypertensives and controls
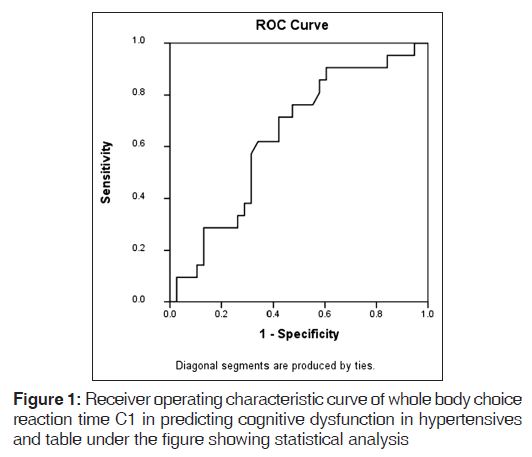
Delayed VSRTs and VCRTs were observed in hypertensives compared with controls (P = 0.07 and 0.02, respectively). Choice RTs were more delayed. WBSRT C1, WBSRT C2 and WBSRT C2 - C1 were delayed in hypertensives compared with controls, and were statistically significant (P = 0.01, 0.003 and 0.005, respectively). WBCRT C1, WBCRT C2 and WBCRT C2 - C1 were delayed in hypertensives compared with controls, and were statistically significant (P = 0.05, <0.001 and 0.07, respectively). Choice RTs were more delayed than simple RTs [Table 2]. Our emphasis was more on recording WBCRT C1, which approximately measured the time required for central processing, i.e., cognition. WBCRT C1 was more delayed compared with WBSRT C1, suggesting that central processing was delayed in hypertensives. The ROC curve of WBCRT C1, when predicting cognitive dysfunction in hypertensive patients, was constructed and the AUC was found to be 0.640 (95% CI); this was statistically insignificant (P = 0.0.07). The best cut-off values for WBCRT C1 when predicting cognitive dysfunction in hypertensive patients were 538.5 (sensitivity 76.2%; specificity 50%) [Figure 1].
| Variables | Group 1 hypertension n=60 Mean (SD) |
Group 2 controls n=60 Mean (SD) |
t value | P value |
|---|---|---|---|---|
| VSRT (ms) | 291.8 (57.9) | 281 (57.9) | −1.0058 | 0.31 |
| VCRT (ms) | 345 (84.8) | 332.4 (68.9) | −0.8875 | 0.37 |
| WBSRT C1 (ms) | 411.1 (85) | 408.6 (88) | −0.1563 | 0.87 |
| WBSRT C2 (ms) | 729.9 (115) | 705.3 (113) | −1.1690 | 0.24 |
| WBSRT C2-C1 | 318.8 (73.6) | 296.7 (80.5) | −1.5587 | 0.12 |
| WBCRT C1 (ms) | 562.6 (108) | 523.5 (98.8) | −2.0458 | 0.04* |
| WBCRT C2 (ms) | 948.6 (172) | 875.6 (129.9) | −2.5948 | 0.01* |
| WBCRT C2-C1 | 386 (95.3) | 352.1 (79.7) | −2.0924 | 0.03* |
*P<0.05, VSRT: Visual simple reaction time, VCRT: Visual choice reaction time, WBSRT: Whole body simple reaction time, WBCRT: Whole body choice reaction time, C1: Chronoccopic reading 1, C2: Chronoscopic reading 2
Table 2: Comparison of reaction times among hypertensives and controls
Discussion
We observed that RTs are delayed in hypertensives. WBCRT C1was significantly delayed in hypertensives, which indicates involvement of cognition. We found that WBRT C1 can be predictive of cognitive dysfunction in hypertensives.
There are several limitations of the study. Although controls were age and sex matched, their BMI were not matching. It is known that BMI affects cognition. Another limitation of our study was that we did not perform the gold standard test that could identify cognitive dysfunction so that we could compare our findings and assess the sensitivity and specificity of the test. However, batteries of tests are time consuming and require skilled staff. On the contrary, RTs can be easily performed on an OPD basis. They can be sensitive indicators of cognitive dysfunction, especially attention and psychomotor speed. Therefore, the strength of our study was that we could use RTs as a screening tool for early detection of cognitive dysfunction.
| WBCRT C1 | Asymptotic 95% confidence interval |
|||
|---|---|---|---|---|
| Area | Std. errora |
Asymptotic sig.b |
Lower bound |
Upper bound |
| 0.640 | 0.07 | 0.07 | 0.49 | 0.78 |
WBCRT: Whole body choice reaction time chronoscopic reading 1. The test result variable(s): WBCRT C1 has at least one tie between the positive actual state group and the negative actual state group. Statistics may be biased. a. Under the non-parametric assumption, b. Null hypothesis: true area=0.5
Area under the curve
Mental activities involved in the acquisition, storage, retrieval and use of information are referred to here by the term cognition.[13] An increased risk of declining cognitive function with aging is well known, especially with regard to working memory, information processing speed and long-term memory.[14,15] Information processing speed is an important resource, defined by the time parameters of a specific cognitive task. Shortest latencies are usually associated with youth and better performances. The label “cognitive slowing” has been applied to increases in those latencies, which in turn can be held responsible for many aspects of declining cognitive functioning. Hypertension is known to cause cognitive dysfunction. Many studies using neuropsychological batteries of tests have shown that hypertension affects cognition.[16,17] Some studies on hypertension have indicated a decline in certain cognitive domains.[18-22] RT measurement includes the latency in the sensory neural code traversing the peripheral and central pathways; perceptive, cognitive, volitional processing. In choice RT, the time required for central processing increases whereas the time required for peripheral response does not alter much. At this point, we require a tool that clearly measures the time required for central processing and peripheral processing in total RT. Many studies shown delay in visual and auditory simple and choice RTs in hypertension, but they have failed to explain whether the delay was because of central processing or time taken for peripheral response. In our study, along with VSRT and VCRT, we have measured WBSRT and WBCRT, in which WBCRT C1 apparently measures the time required for perception and cognition, the time taken for lifting the foot from the onset of stimulus from starting board. WBCRT C2 apparently measures motor signals traversing both central and peripheral neuronal structures, the total time required for placing the foot on the stepping board from onset of stimulus. Therefore, it becomes easy to tease out the central effect versus the peripheral effects when RTs are slowed. The focus of our study was to measure the WBCRT C1 in hypertensives and compare it with controls, which approximately indicates the difference in cognition between these two groups. We hypothesised that WBCRT C1 can be used as a screening tool to detect cognitive dysfunction.
In the present study, visual RTs are delayed in hypertension. Choice RTs were more delayed, indicating that cognition is affected. Both WBCRT C1 and WBCRT C2 were delayed in hypertension, which indicates that there is involvement of both central processing, i.e., cognition and peripheral response. WBCRT C1 was more delayed than WBSRT C1 in hypertension, which again indicates that cognition is involved.
Whole body RTs were delayed to a greater extent in hypertensives compared with visual RTs. Therefore, we can say that whole body RT measurement is more sensitive. This could be due to the different representational areas that are supplied by different vessels. The anterior central artery supplies the area representing the hand (visual auditory RTs) and the posterior central artery supplies the area representing the legs (whole body RT). Further studies should be done to elucidate why, in hypertension, the anterior cerebral artery is affected earlier. There was no significant correlation between duration of hypertension and WBCRT C1 (r = 0.229). The reason could be that all patients were on antihypertensive medication. The ROC curve of WBCRT C1, predicting cognitive dysfunction in hypertensive patients, was constructed and the best cut-off value was 538.5 (sensitivity 76.2%; specificity 50%). Alhough the sensitivity and specificity of the test were not enough to use this test as a screening tool, future studies with well-matched controls may provide insight to its utility. One of the reasons for the low specific value was that all hypertensives were on antihypertensive treatment.
There are no systemic reviews implicating that RTs, especially WBCRT C1, can detect cognitive dysfunction. WBCRT C1 can apparently measure the time required for cognition, although not accurately. This study may provide a platform for further studies in this direction, particularly the underlying mechanisms with properly matched controls.
From this study, we can conclude that hypertension does affect the RT, whereby severity of slowing may be related to difficulty of the task and prevalence of central and peripheral nerve deficits seen as side-effects of hypertension. Auditory, visual RTs, the simplest of tasks with shortest path between peripheral and central nervous system, showed less delayed RTs. Choice visual RTs will be more delayed because of involvement of complicated circuits. When a more complicated task included detecting movement, signal transmission and interpretation, as in whole body RTs, a significant difference was seen. With choice whole body RTs, the difference increased. This difference is attributed to cognition. In whole body RT with chronoscopic reading C1 and C2 - C1, probably, it is possible to say how much of time is required for cognition and how much of time is required for motor response.
Source of Support
Nil.
Conflict of Interest
None declared.
References
- Birns J, Kalra L. Cognitive function and hypertension. J Hum Hypertens 2009;23:86-96.
- Robbins MA, Elias MF, Elias PK, Budge MM. The maine-syracuse study. Blood pressure and cognitive function in an African-American and a Caucasian-American sample. Psychosom Med 2005;67:707-14.
- Elias MF, Wolf PA, D’Agostino RB, Cobb J, White LR. Untreated blood pressure level is inversely related to cognitive functioning: The Framingham Study. Am J Epidemiol 1993;138:353-64.
- Elias MF, Sullivan LM, Elias PK, D’Agostino RB Sr, Wolf PA, Seshadri S, et al. Left ventricular mass, blood pressure, and lowered cognitive performance in the Framingham offspring. Hypertension 2007;49:439-45.
- Launer LJ, Masaki K, Petrovich H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late-life cognitive function. The Honolulu-Asia Aging Study. JAMA 1995;274:1846-51.
- Elias PK, Elias MF, Robbins MA, Budge MM. Blood pressure-related cognitive decline: Does age make a difference? Hypertension 2004;44:631-6.
- Waldstein SR. Hypertension and neuropsychological function: A lifespan perspective. Exp Aging Res 1995;21:321-52.
- Wilkie F, Eisdorfer C. Intelligence and blood pressure in the aged. Science 1971;172:959-62.
- Elias MF, D’Agostiono RB, Elias PK, Wolf PA. Neuropsychological test performance, cognitive functioning, blood pressure, and age: The Framingham Heart Study. Exp Aging Res 1995;21:369-91.
- Kovács KR, Szekeres CC, Bajkó Z, Csapó K, Molnár S, Oláh L, et al. Cerebro- and cardiovascular reactivity and neuropsychological performance in hypertensive patients. J Neurol Sci 2010;299:120-5.
- Chiaravalloti ND, Christodoulou C, Demaree HA, DeLuca J. Differentiating simple versus complex processing speed: Influence on new learning and memory performance. J Clin Exp Neuropsychol 2003;25:489-501.
- Steinmetz J, Rasmussen LS; ISPOCD GROUP. Choice reaction time in patients with post-operative cognitive dysfunction. Acta Anaesthesiol Scand 2008;52:95-8.
- Smith JW, Evans AT, Costall B, Smythe JW. Thyroid hormones, brain function and cognition: A brief review. Neurosci Biobehav Rev 2002;26:45-60.
- Mariotti S, Franceschi C, Cossarizza A, Pinchera A. The aging thyroid. Endocr Rev 1995;16:686-715.
- Davis JD, Stern RA, Flashman LA. Cognitive and neuropsychiatric aspects of subclinical hypothyroidism: Significance in the elderly. Curr Psychiatry Rep 2003;5:384-90.
- O’Brien JT. Vascular cognitive impairment. Am J Geriatr Psychiatry 2006;14:724-33.
- Bowler JV. Vascular cognitive impairment. Stroke 2004;35:386-8.
- Carey CL, Kramer JH, Josephson SA, Mungas D, Reed BR, Schuff N, et al. Subcortical lacunes are associated with executive dysfunction in cognitively normal elderly. Stroke 2008;39:397-402.
- Tomlinson BE, Blessed G, Roth M. Observations on the brains of demented old people. J Neurol Sci 1970;11:205-42.
- Erkinjuntti T, Haltia M, Palo J, Sulkava R, Paetau A. Accuracy of the clinical diagnosis of vascular dementia: A prospective clinical and post-mortem neuropathological study. J Neurol Neurosurg Psychiatry 1988;51:1037-44.
- Skoog I, Berg S, Johansson B, Palmertz B, Andreasson LA. The influence of white matter lesions on neuropsychological functioning in demented and non-demented 85-year-olds. Acta Neurol Scand 1996;93:142-8.
- van Swieten JC, Geyskes GG, Derix MM, Peeck BM, Ramos LM, van Latum JC, et al. Hypertension in the elderly is associated with white matter lesions and cognitive decline. Ann Neurol 1991;30:825-30.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.