Clinical Evaluation for the Thrombopoietic Activity of Platenza Tablet in Cases of Dengue with Thrombocytopenia - Randomized Open Label Comparative Clinical Study
2 Department of General Medicine, Patna Medical College and Hospital, Patna, India
Citation: Srikrishna HA, et al. Clinical Evaluation for the Thrombopoietic Activity of Platenza Tablet in Cases of Dengue with Thrombocytopenia - Randomized Open Label Comparative Clinical Study. Ann Med Health Sci Res. 2018;8:29-38
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Introduction: Dengue is one of the vector borne viral infections growing across various countries. Despite the availability of various therapeutic agents, a potentially safe and efficacious herbal formulation is of high need. Objective: To evaluate the safety and efficacy of platenza tablets in increasing the platelet counts in cases of dengue fever associated with thrombocytopenia. Method: This study was conducted at Patna Medical College and Hospital, the Department of Medicine, Bihar. It was a randomized open label controlled comparative clinical study. Forty subjects were taken up for the study and grouped into platenza group (n=21) and standard comparator group (n=19) where platenza group received platenza tablets at a dose of two tablets twice daily oral and standard comparator received Carica papaya leaves extract at a dose of one tablet three times daily for a period of 10 days. Results: Platelet count was monitored at screening, and on each day from Day 1 to Day 4, Day 6, Day 8 and Day 10. Post 10 days, it was observed that, platenza group showed a potential increase in platelet count from 52905 to 207381 cells/mm3 compared to that of the standard comparator group. There were no drop outs in the study. Conclusion: Based on the improvement in subjective and objective parameters; it can be concluded that platenza tablets compared to the Carica papaya leaves extract alone, exhibited a potential Thrombopoietic activity along with the reduction of clinical signs and symptoms of dengue fever associated with thrombocytopenia.
Keywords
Dengue fever; Carica papaya leaves; Platelets
Introduction
Dengue fever, also known as break-bone fever, is an infectious tropical disease caused by the dengue virus. Symptoms include fever, headache, muscle and joint pains, and a characteristic skin rash. In a small number of cases, the disease develops into the life-threatening dengue hemorrhagic fever, resulting in bleeding, low levels of blood platelets and plasma leakage or into dengue shock syndrome, where dangerously low blood pressure occurs. [1,2]
Presently there is no approved vaccine or drug against this dengue virus although various researches are ongoing and all the therapeutic agents are in developmental phase. [3]. Therefore, there is an urgent need of development of alternative solutions for dengue. Several plant species have been reported with anti-dengue activity. Recently, the use of alternative medicine and the consumption of plant materials have increased across various countries in the world of which Carica papaya had been one which has underwent various scientific investigations to evaluate the biological activities of various parts of Carica papaya including roots or latex, fruits, leaves, rind, seeds. The leaves of papaya have been shown to contain many active components that can increase the total antioxidant activity in blood and reduce lipid peroxidation level, such as papain and chymopapain, cystatin, tocopherol, ascorbic acid, flavonoids, cyanogenic- glucosides and glucosinolates.
Various case reports showed that leaves extract of Carica papaya taken continuously for five consecutive days twice daily increased the platelet counts, leukocyte counts and neutrophil counts compared with before the treatment. [1] Considering the pathophysiology impact and the visceral involvement in this clinical condition, in this study we observed the enhanced potential efficacy of platenza tablets consisting Carica papaya leaf extract along with Phyllanthus maderaspatensis, [4] Piper nigrum [5,6] and Tinospora cordifolia [7] compared to only leaves extract of Carica papaya in increasing the platelet count along with symptomatic relief in dengue fever associated with thrombocytopenia.
Aim of the study
To clinically evaluate the efficacy of Platenza Tablets in normalizing the platelet counts in cases of dengue fever associated with Thrombocytopenia as a comparative study with standard comparator.
Materials and Methods
Materials
Platenza group: This group receives platenza tablets, a polyherbal formulation consisting of: Carica papaya, Piper nigrum, Phyllanthus madraspatensis, Tinospora cordifolia at a dose of 2 tablets twice daily oral for a duration of 10 days.
Standard comparator group: This group receives standard comparator tablets consisting of leaves extract of Eranda Karkati (Carica Papaya) at a dosage of 1 tablet three times daily oral for a period of 10 days.
This study was performed during August 2017.
Clinical Study
Design: This study was approved by the institutional Ethics Committee of Patna Medical College and Hospital. This was an open label randomized phase II comparative clinical study conducted at the inpatient department of internal medicine, Patna Medical College and Hospital, Bihar.
Methods: Forty eligible subjects who met the inclusion criteria were grouped into platenza (n=21) and Standard comparator group (n=19). Platenza group received two tablets twice daily orally for a period of 10 days and standard comparator group received one tablet thrice daily orally for a period of 10 days. Platelet count was determined in the clinical hospital laboratory, during the hospitalization period at screening, and on each day from Day 1 to Day 4, Day 6, Day 8 and Day 10. Clinical parameters were assessed from the day of screening, at the end of day 3, day 6 and day 10 and the collected data was taken for evaluation.
Selection Criteria: The study protocol, CRFs, regulatory clearance documents, product related information and informed consent form (in English & Hindi) were submitted to the Institutional Ethics Committee and were approved by the same. Informed consent was explained to the subjects and they agreed to participate by signing a written consent form. Forty subjects were selected for the study using the following criterias.
Inclusion Criteria: Both male and female subjects between the age group of ≥18 years to ≤ 60 years who are confirmed to have DF (Dengue Fever) grade 1 and grade 2 by IgG, IgM and NS1 Antigen. Subjects who have been diagnosed with clinically significant dengue fever associated with thrombocytopenia with platelet count between 30000/μL to 1,00,000/μL, subjects with the SGPT level not more than 2 times the upper limit of normal, subjects with stable vitals like pulse and blood pressure, subjects willing to give written informed consent, subjects who had not participated in similar kind of study in last 3 months were included into the study.
Exclusion Criteria: Subjects diagnosed with DHF Grade 3 or 4, with hypotension / hypovolemia, subjects with platelet count <30000/μL, subjects presenting with hemorrhagic phenomena at screening evidenced with positive torniquette test, petechiae, ecchymoses, or purpura, bleeding from the oral mucosa, gastrointestinal tract, injection sites or other locations and haemetemesis or melaena, subjects with dengue fever associated with any bleeding disorders or those taking blood thinning medications such as aspirin or warfarin, subjects with the history of any severe metabolic disorders, pregnant and lactating women, subjects not willing to sign informed consent form are excluded from the study.
Statistical Analysis
The data were presented in terms of mean and standard deviation (SD), analyzed by descriptive and inferential statistics and all analysis was made using the GraphPad Prism Version 6.07 for windows, GraphPad Software, San Diego, California, United States. www.graphpad.com and P value <0.05 was considered statistically significant.
Results
The trial population consisted of adult subjects diagnosed with dengue fever associated with thrombocytopenia who were hospitalized in the internal medicine ward in the month of August 2017. Before the study, after screening and according to the inclusion criteria only 40 patients were recognized eligible and were randomized into two groups, 21 cases in Platenza group and 19 cases in standard comparator group. Table 1 gives the analysis of demographic data, Table 2 gives the assessment of clinical parameters based on Score 0-3 was assessed at Screening, end of Day 3, Day 6, and Day 10 as compared to the values at baseline and Standard comparator group. Table 3 shows the analysis of Study specific efficacy parameters assessed from Screening, at the end of Day 1, Day 2, Day 3, Day 4, Day 6, Day 8 and Day 10 as compared to baseline and Standard Comparator Group. Table 4 shows the analysis for safety parameters based on the hematological and biochemical investigations assessed at Screening/Baseline and Standard Comparator Group. Table 5 shows the assessment of Quality of Life from Screening and at the end of Day 10 (End of the study). Table 6 shows the overall assessment at the end of the study compared to Screening and to that of the standard comparator group.
| Demographic Data | Platenza Group | Standard Comparator Group |
|---|---|---|
| No. of Subjects | 21 | 19 |
| Gender(Male: Female) | (12:09) | (06:13) |
| Diet (Veg: Non Veg) | (07:14) | (07:12) |
| Age | 28.7 ± 6.6 | 30.3 ± 5.9 |
| Marital status (Married: Unmarried) | (10:11) | (15:04) |
Table 1: Analysis of demographic data.
| Clinical Symptoms | Scheduled Visits | Platenza Group | Standard Comparator Group |
|---|---|---|---|
| Temperature (in °F) | Screening/Baseline | 101.8 ± 1.12 | 102.3 ± 0.71 |
| Day 3 | 100.5 ± 1.07 | 101.3 ± 0.66 | |
| q:p<0.0096 | |||
| Day 6 | 98.45 ± 0.53 | 100.1 ± 1.05 | |
| q:p< 0.0001 | |||
| a:p<0.0001 | a:p<0.0001 | ||
| Day 10 | 98.15 ± 0.44 | 98.93 ± 1.02 | |
| q:p<0.0027 | |||
| a:p<0.0001 | a:p<0.0001 | ||
| Fever | Screening/Baseline | 3 ± 0.32 | 3 ± 0 |
| Day 3 | 2.05 ± 0.59 | 2.37 ± 0.6 | |
| Day 6 | 0.32 ± 0.67 | 0.89 ± 0.81 | |
| a:p<0.0001 | a:p<0.0001 | ||
| q:p<0.019 | |||
| Day 10 | 0.14 ± 0.48 | 0.37 ± 0.76 | |
| a:p<0.0001 | a:p<0.0001 | ||
| Arthralgia | Screening/Baseline | 0.38 ± 0.97 | 0.53 ± 0.96 |
| Day 3 | 0.33 ± 0.86 | 0.37 ± 0.76 | |
| Day 6 | 0.19 ± 0.51 | 0.21 ± 0.42 | |
| Day 10 | 0.1 ± 0.44 | 0.05 ± 0.23 | |
| Myalgia | Screening/Baseline | 2.19 ± 0.93 | 2.37 ± 0.96 |
| Day 3 | 1.52 ± 0.87 | 1.95 ± 0.85 | |
| Day 6 | 0.52 ± 0.68 | 1.26 ± 0.65 | |
| a:p<0.0001 | a:p<0.0016 | ||
| Day 10 | 0.14 ± 0.36 | 0.37 ± 0.68 | |
| a:p<0.0001 | a:p<0.0001 | ||
| Headache | Screening/Baseline | 2 ± 1.38 | 1.9 ± 1.37 |
| Day 3 | 1.29 ± 1.1 | 1.53 ± 1.17 | |
| Day 6 | 0.57 ± 0.75 | 0.84 ± 0.76 | |
| a:p<0.0032 | a:p<0.0189 | ||
| Day 10 | 0.14 ± 0.36 | 0.16 ± 0.37 | |
| a:p<0.0001 | a:p<0.0001 | ||
| Loss of Appetite | Screening/Baseline | 0.43 ± 1.12 | 0.26 ± 0.81 |
| Day 3 | 0.24 ± 0.7 | 0.26 ± 0.81 | |
| Day 6 | 0.19 ± 0.51 | 0.21 ± 0.63 | |
| Day 10 | 0.05 ± 0.22 | 0.16 ± 0.5 |
Between the Platenza group analysis.
Statistical test: Mann Whitney test & Unpaired t test
a: as compared to Screening/Baseline
q: as compared to Standard Comparator
Significance was fixed at p<0.05
Table 2: Assessment of clinical parameters.
| Study Specific Efficacy Parameters | Scheduled Visits | Platenza Group | Standard Comparator Group |
|---|---|---|---|
| Platelet cells/mm3 | Screening/Baseline | 52905 ± 21377 | 53842 ± 17436 |
| Day 1 | 58286 ± 20073 | 58789 ± 16390 | |
| a:p<0.0001 | a:p<0.0294 | ||
| Day 2 | 68810 ± 21205 | 64158 ± 16153 | |
| a:p<0.0001 | a:p<0.0006 | ||
| Day 3 | 86571 ± 26086 | 76684 ± 19553 | |
| a:p<0.0001 | a:p<0.0004 | ||
| Day 4 | 103524 ± 29063 | 92053 ± 31283 | |
| a:p<0.0001 | a:p<0.0018 | ||
| Day 6 | 135024 ± 38159 | 104105 ± 37169 | |
| q:p<0.0135 | |||
| a:p<0.0001 | a:p<0.001 | ||
| Day 8 | 167429 ± 47892 | 122474 ± 55116 | |
| q:p<0.0088 | |||
| a:p<0.0001 | a:p<0.0035 | ||
| Day 10 | 207381 ± 46534 | 141368 ± 57521 | |
| q:p<0.0003 | |||
| a:p<0.0001 | a:p<0.0003 | ||
| Hematocrit % | Screening/Baseline | 40.29 ± 4.17 | 43.05 ± 5.74 |
| Day 1 | 40.1 ± 5.09 | 42.79 ± 4.08 | |
| Day 2 | 38.76 ± 4.49 | 41.79 ± 4.1 | |
| q:p<0.0327 | |||
| Day 3 | 37.71 ± 3.85 | 41.11 ± 4.4 | |
| q:p<0.0132 | |||
| Day 4 | 36.95 ± 3.78 | 39.95 ± 3.47 | |
| q:p<0.0131 | |||
| Day 6 | 36.52 ± 3.83 | 38.95 ± 3.87 | |
| a:p<0.0284 | a:p<0.0231 | ||
| Day 8 | 36.57 ± 4.74 | 38.89 ± 4.12 | |
| a:p<0.0308 | |||
| Day 10 | 36.57 ± 4.15 | 38.84 ± 4.18 | |
| a:p<0.0287 | |||
Prothrombin Time (seconds) |
Screening/Baseline | 11.71 ± 1.59 | 11.16 ± 1.17 |
| Day 1 | 11.62 ± 1.24 | 11.47 ± 1.26 | |
| Day 2 | 11.43 ± 1.21 | 11.63 ± 1.17 | |
| Day 3 | 11.86 ± 1.06 | 11.84 ± 1.02 | |
| a:p<0.0159 | |||
| Day 4 | 12.19 ± 1.08 | 12.05 ± 0.91 | |
| Day 6 | 12.19 ± 1.03 | 11.89 ± 0.94 | |
| Day 8 | 12.48 ± 1.08 | 12.26 ± 0.73 | |
| a:p<0.0069 | |||
| Day 10 | 12 ± 1.04 | 11.97 ± 0.72 | |
| Clotting Time (minutes) | Screening/Baseline | 3.61 ± 0.94 | 3.94 ± 1.25 |
| Day 1 | 3.69 ± 0.88 | 3.91 ± 1.17 | |
| Day 2 | 3.83 ± 0.67 | 3.85 ± 1.09 | |
| Day 3 | 3.96 ± 0.62 | 3.83 ± 1.1 | |
| Day 4 | 3.67 ± 0.69 | 3.83 ± 1.15 | |
| Day 6 | 3.69 ± 0.75 | 3.93 ± 1 | |
| Day 8 | 3.6 ± 0.72 | 4 ± 0.95 | |
| Day 10 | 3.65 ± 0.72 | 4.1 ± 0.93 | |
| Bleeding Time (minutes) | Screening/Baseline | 2.16 ± 1.01 | 2.39 ± 1.04 |
| Day 1 | 2.16 ± 1.01 | 2.21 ± 0.98 | |
| Day 2 | 2.14 ± 1.02 | 2.21 ± 0.84 | |
| Day 3 | 2.09 ± 0.95 | 2.24 ± 0.97 | |
| Day 4 | 2.16 ± 1.06 | 2.34 ± 0.95 | |
| Day 6 | 2.17 ± 1.02 | 2.41 ± 1.03 | |
| Day 8 | 2.18 ± 1.01 | 2.44 ± 1.01 | |
| Day 10 | 2.17 ± 1.02 | 2.41 ± 1.03 |
Within the Platenza group analysis
Statistical test: Repeated measures ANOVA followed by Tukey's multiple comparisons test
a: as compared to Day 1 (screening/baseline)
Between the Platenza group analysis
Statistical test: Unpaired t test
q: as compared to Standard Comparator Group
Table 3: Assessment of study specific efficacy parameters.
| Laboratory Investigations | Scheduled Visits | Platenza Group | Standard Comparator Group |
|---|---|---|---|
| Hemoglobin (%) | Screening/baseline | 11 ± 2.1 | 10.71 ± 1.47 |
| Day 10 | 11.71 ± 1.89 | 10.99 ± 1.31 | |
| a:p<0.0001 | a:p<0.0436 | ||
| Total WBC count cells/mm3 | Screening/baseline | 7143 ± 815.2 | 7547 ± 1016 |
| Day 10 | 7181 ± 696.1 | 7226 ± 629.7 | |
| Neutrophils cells/ mm3 | Day 1 (screening/baseline) | 70 ± 3.55 | 70.26 ± 5.02 |
| Day 10 | 66.86 ± 3.53 | 66.89 ± 5.36 | |
| a:p<0.0005 | a:p<0.0011 | ||
| Lymphocytes cells/ mm3 | Screening/baseline | 25.9 ± 4.05 | 25.63 ± 4.15 |
| Day 10 | 28.71 ± 3.72 | 28.26 ± 3.63 | |
| a:p<0.0024 | a:p<0.0016 | ||
| Eosinophils cells/ mm3 | Screening/baseline | 2.52 ± 1.08 | 2.58 ± 1.54 |
| Day 10 | 2.57 ± 1.17 | 2.58 ± 1.74 | |
| Monocytes cells/ mm3 | Screening/baseline | 1.43 ± 0.93 | 1.63 ± 0.76 |
| Day 10 | 1.48 ± 0.87 | 1.95 ± 0.78 | |
| Basophils cells/ mm3 | Screening/baseline | 0.76 ± 0.77 | 0.83 ± 0.92 |
| Day 10 | 1.1 ± 0.77 | 1.22 ± 1.22 | |
| ESR mm/hr | Screening/baseline | 13.62 ± 2.5 | 13.68 ± 3.15 |
| Day 10 | 10.38 ± 3.22 | 11.79 ± 4.2 | |
| a:p<0.0001 | a:p<0.0079 | ||
| SGPT IU/L | Screening/baseline | 32.62 ± 9 | 35.67 ± 8.11 |
| Day 10 | 27.52 ± 10.4 | 28.11 ± 8.5 | |
| a:p<0.0211 | a:p<0.007 | ||
| Serum Creatinine mg/dl | Screening/baseline | 0.78 ± 0.13 | 0.79 ± 0.13 |
| Day 10 | 0.66 ± 0.09 | 0.7 ± 0.11 | |
| a:p<0.0013 | a:p<0.0083 |
Within the Platenza group analysis
Statistical test: Paired t test
a: as compared to Screening/Baseline
Table 4: Assessment of hematological parameters.
| Questionnaire to assess quality of Life | Scale | Platenza Group | Platenza Group | Standard Comparator Group | Standard Comparator Group | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Screening | % | At End of Study | % | Screening | % | At End of Study | % | ||||
| 1) Do you feel tired or fatigued? | Always=1 | 3 | 14 | 0 | 0 | 1 | 5 | 0 | 0 | ||
| Often=2 | 6 | 29 | 0 | 0 | 9 | 47 | 0 | 0 | |||
| Sometimes=3 | 12 | 57 | 15 | 71 | 9 | 47 | 16 | 84 | |||
| Never=4 | 0 | 0 | 6 | 29 | 0 | 0 | 3 | 16 | |||
| 2) Do you feel week? | Always=1 | 3 | 14 | 0 | 0 | 1 | 5 | 0 | 0 | ||
| Often=2 | 15 | 71 | 0 | 0 | 11 | 58 | 0 | 0 | |||
| Sometimes=3 | 3 | 14 | 13 | 62 | 7 | 37 | 17 | 89 | |||
| Never=4 | 0 | 0 | 8 | 38 | 0 | 0 | 2 | 11 | |||
| 3) Does your skin look pale? | Always=1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Often=2 | 13 | 62 | 0 | 0 | 9 | 47 | 2 | 11 | |||
| Sometimes=3 | 5 | 24 | 14 | 67 | 3 | 16 | 7 | 37 | |||
| Never=4 | 3 | 14 | 7 | 33 | 7 | 37 | 10 | 53 | |||
| 4) Do you get short of breath? | Always=1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Often=2 | 6 | 29 | 0 | 0 | 9 | 47 | 0 | 0 | |||
| Sometimes=3 | 12 | 57 | 4 | 19 | 5 | 26 | 10 | 53 | |||
| Never=4 | 3 | 14 | 17 | 81 | 5 | 26 | 9 | 47 | |||
| 5) Do you get dizzy? | Always=1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Often=2 | 13 | 62 | 0 | 0 | 8 | 42 | 0 | 0 | |||
| Sometimes=3 | 7 | 33 | 2 | 10 | 11 | 58 | 10 | 53 | |||
| Never=4 | 1 | 5 | 19 | 90 | 0 | 0 | 9 | 47 | |||
6) Is it difficult to concentrate? |
Always=1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Often=2 | 13 | 62 | 0 | 0 | 9 | 47 | 2 | 11 | |||
| Sometimes=3 | 8 | 38 | 15 | 71 | 10 | 53 | 11 | 58 | |||
| Never=4 | 0 | 0 | 6 | 29 | 0 | 0 | 6 | 32 | |||
| 7) Have you experienced a rapid heartbeat? | Always=1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Often=2 | 13 | 62 | 0 | 0 | 8 | 42 | 1 | 5 | |||
| Sometimes=3 | 7 | 33 | 8 | 38 | 10 | 53 | 9 | 47 | |||
| Never=4 | 1 | 5 | 13 | 62 | 1 | 5 | 9 | 47 | |||
| 8) Is your menstrual cycle irregular? | Always=1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Often=2 | 2 | 22 | 4 | 44 | 1 | 8 | 0 | 0 | |||
| Sometimes=3 | 4 | 44 | 2 | 22 | 9 | 69 | 10 | 77 | |||
| Never=4 | 3 | 33 | 3 | 33 | 3 | 23 | 3 | 23 | |||
| 9) Do you have numbness or coldness in your hands or feets? | Always=1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Often=2 | 8 | 38 | 0 | 0 | 10 | 53 | 0 | 0 | |||
| Sometimes=3 | 10 | 48 | 5 | 24 | 9 | 47 | 13 | 68 | |||
| Never=4 | 3 | 14 | 16 | 76 | 0 | 0 | 6 | 32 | |||
| 10) Are you irritable? | Always=1 | 1 | 5 | 0 | 0 | 1 | 5 | 0 | 0 | ||
| Often=2 | 7 | 33 | 0 | 0 | 12 | 63 | 0 | 0 | |||
| Sometimes=3 | 11 | 52 | 12 | 57 | 6 | 32 | 18 | 95 | |||
| Never=4 | 2 | 10 | 9 | 43 | 0 | 0 | 1 | 5 | |||
| 11) Do you feel sad or depressed? | Always=1 | 1 | 5 | 0 | 0 | 1 | 5 | 0 | 0 | ||
| Often=2 | 4 | 19 | 2 | 10 | 4 | 21 | 2 | 11 | |||
| Sometimes=3 | 15 | 71 | 7 | 33 | 13 | 68 | 14 | 74 | |||
| Never=4 | 1 | 5 | 12 | 57 | 1 | 5 | 3 | 16 | |||
| 12) Do you know what if your platelet count is below 150000 per microlitre of blood? | Yes | 4 | 19 | 16 | 76 | 6 | 32 | 15 | 79 | ||
| No | 17 | 81 | 5 | 24 | 13 | 68 | 4 | 21 | |||
| 13) Have you ever been told that you have Dengue? | Yes | 10 | 48 | 20 | 95 | 10 | 53 | 19 | 100 | ||
| No | 11 | 52 | 1 | 5 | 9 | 47 | 0 | 0 | |||
Table 5: Quality of life assessment.
| Overall Impression | By the Investigator | % Subjects | By the Investigator | % Subjects |
|---|---|---|---|---|
| Cured | 11 | 52% | 6 | 32% |
| Marked improvement | 9 | 43% | 3 | 16% |
| Moderate improvement | 1 | 5% | 6 | 32% |
| Slight improvement | 0 | 0% | 4 | 21% |
Table 6: Overall assessment of the study.
Among the total 40 cases; 21 cases in Platenza group and 19 cased in Standard comparator group were enrolled into the study. In Platenza group, among the 21 cases, 12 subjects were male and 9 were females aged between 28.7 ± 6.6. In Standard comparator group, among the 19 cases, 06 subjects were male and 13 subjects were female aged between 30.3 ± 5.9 years [Table 1].
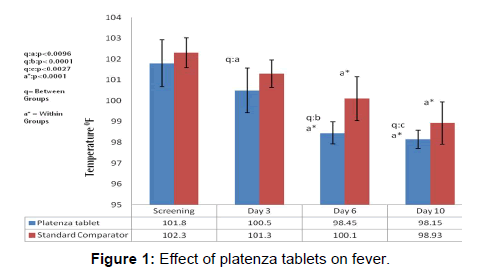
The efficacy of platenza tablets on fever is graphically represented in Figure 1. In Platenza group, the mean fever expressed in terms of temperature (°F) at Screening was 101.8 ± 1.12 which reduced to 100.5 ± 1.07 at Day 3 at a significance level of p<0.0096 compared to that of the Standard comparator group, 98.45 ± 0.53, at Day 6 at a significance level of p< 0.0001 compared to that of the Standard comparator group and p<0.0001 compared to that of the baseline, 98.15 ± 0.44 at Day 10 at a significance level of p<0.0027 compared to that of the Standard comparator group and p<0.0001 compared to that of the baseline. In Standard comparator group, the mean fever expressed in terms of temperature (°F) at Screening was 102.3 ± 0.71 which reduced to 101.3 ± 0.66 at Day 3, 100.1 ± 1.05 at Day 6 at a significance level of p<0.0001 compared to that of baseline, 98.93 ± 1.02 at Day 10 at a significance level of p<0.0001 compared to that of baseline [Table 2].
In Platenza group, the mean Fever score at Screening was 3 ± 0.32, which reduced to 2.05 ± 0.59 at Day 3, 0.32 ± 0.67 at Day 6 at a significant level of a:p<0.0001 as compared to baseline and at a significance level of p<0.019 compared to that of the Standard comparator group, and 0.14 ± 0.48 at Day 10 at a significance level of a:p<0.0001 compared to that of baseline respectively. In Standard comparator group, mean fever score at screening was 3 ± 0 which reduced to 2.37 ± 0.6 at Day 3, 0.89 ± 0.81 at Day 6 at a significance level of a:p<0.0001 compared to that of baseline and 0.37 ± 0.76 at Day 10 at a significance level of a:p<0.0001 compared to that of baseline respectively. Scoring of fever was done on a scale of 0-3 where 0 ≤ 99°F, 1 ≥ 99-100°F, 2≥ 101-102°F, 3 ≥ 102°F.
In Platenza group, mean Arthralgia score at Screening was 0.38 ± 0.97 which reduced to 0.33 ± 0.86 at Day 3, 0.19 ± 0.51 at Day 6 and 0.1 ± 0.44 at Day 10. At Day 10, although there was a trend of improvement, results were not statistically significant compared to neither the baseline nor the Standard comparator group. In Standard comparator group, mean Arthralgia score at screening was 0.53 ± 0.96 which reduced to 0.37 ± 0.76 at Day 3, 0.21 ± 0.42 at Day 6 and 0.05 ± 0.23 at Day 10. At Day 10, although there was a trend of improvement, results were not statistically significant compared to neither the baseline nor the Standard comparator group.
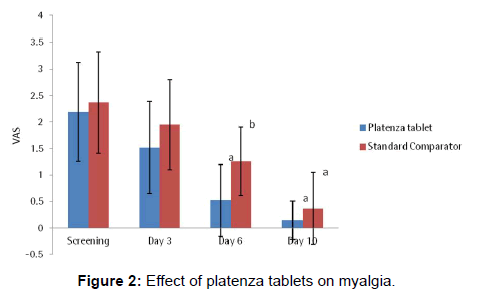
The efficacy of platenza tablets on Myalgia is graphically represented in Figure 2. In Platenza group, mean Myalgia score at Screening was 2.19 ± 0.93 which reduced to 1.52 ± 0.87 at Day 3, 0.52 ± 0.68 at Day 6 at a significance level of p<0.0001 compared to that of the baseline and 0.14 ± 0.36 at Day 10 at a significance level of p<0.0001 compared to that of the baseline. In Standard comparator group, mean Myalgia score at screening was 2.37 ± 0.96, which reduced to 1.95 ± 0.85 at Day 3, 1.26 ± 0.65 at Day 6 at a significance level of p<0.0016 compared to that of the baseline and 0.37 ± 0.68 at Day 10 at a significance level of p<0.0001 compared to that of the baseline.
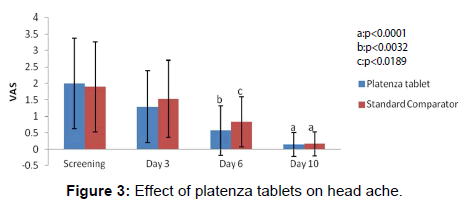
The efficacy of platenza tablets on headache is graphically represented in Figure 3. In Platenza group, mean Headache score at Screening was 2 ± 1.38 which reduced to 1.29 ± 1.1 at Day 3, 0.57 ± 0.75 at Day 6 at a significance level of p<0.0032 compared to that of the baseline and 0.14 ± 0.36 at Day 10 at a significance level of p<0.0001 compared to that of the baseline. In Standard comparator group, mean Headache score at screening was 1.9 ± 1.37 which reduced to 1.53 ± 1.17 at Day 3, 0.84 ± 0.76 at Day 6 at a significance level of p<0.0189 compared to that of the baseline and 0.16 ± 0.37 at Day 10 at a significance level of p<0.0001 compared to that of the baseline.
In Platenza group, mean Loss of Appetite score at Screening was 0.43 ± 1.12 which was increased to 0.24 ± 0.7 at Day 3, 0.19 ± 0.51 at Day 6 and 0.05 ± 0.22 at Day 10. At Day 10, although there was a trend of significant improvement, the results were not statistically significant compared to neither the baseline not the Standard comparator group. In Standard comparator group, mean Loss of Appetite score at screening and Day 3 was 0.26 ± 0.81, which reduced to 0.21 ± 0.63 at Day 6 and 0.16 ± 0.5 at Day 10. At Day 10, although there was a trend of significant improvement, the results were not statistically significant compared to neither the baseline not the Standard comparator group.
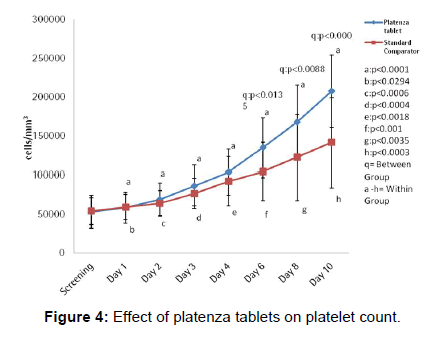
The efficacy of platenza tablets on platelet counts is graphically represented in Figure 4. In Platenza group, mean value for platelet count at screening was 52905 ± 21377 which gradually increased to 58286 ± 20073 (p<0.0001) at Day 1, 68810 ± 21205 (p<0.0001) at Day 2, 86571 ± 26086 (p<0.0001) at Day 3, 103524 ± 29063 (p<0.0001) at Day 4, 135024 ± 38159 (q:p<0.0135) at Day 6 compared to that of the Standard comparator group and p<0.0001 compared to that of the baseline and 167429 ± 47892 (q:p<0.0088) at Day 8 compared to that of the Standard comparator group and p<0.0001 compared to that of the baseline, 207381 ± 46534 (q:p<0.0003) at Day 10 compared to that of the Standard comparator group and p<0.0001 compared to that of the baseline. In Standard comparator group, mean value for Platelet Count at screening was 53842 ± 17436 which increased to 58789 ± 16390 (p<0.0294) at Day 1, 64158 ± 16153 (p<0.0006) at Day 2, 76684 ± 19553 (p<0.0004) at Day 3, 92053 ± 31283 (p<0.0018) at Day 4, 104105 ± 37169 (p<0.001) at Day 6, 122474 ± 55116 (p<0.0035) at Day 8 and 141368 ± 57521 (p<0.0003) at Day 10 compared to that of baseline [Table 3].
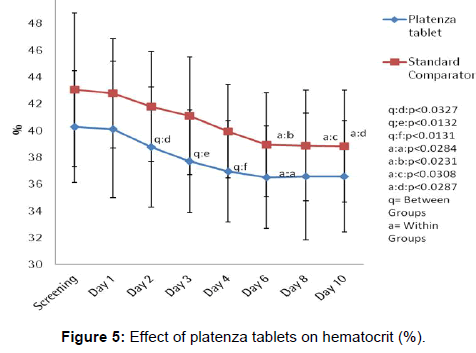
The efficacy of platenza tablets on hematocrit (%) value is graphically represented in Figure 5. In Platenza group, mean percentage of Hematocrit value at Screening was 40.29 ± 4.17 which slightly reduced to 40.1 ± 5.09 at Day 1, further reduced to 38.76 ± 4.49 (q:p<0.0327) at Day 2 compared to that of Standard comparator group, 37.71 ± 3.85 (q:p<0.0132) at Day 3 compared to that of Standard comparator group, 36.95 ± 3.78 (q:p<0.0131) at Day 4 compared to that of Standard comparator group, 36.52 ± 3.83 (p<0.0284) at Day 6 compared to that of baseline, 36.57 ± 4.74 and 36.57 ± 4.15 at Day 8 and Day 10. In Standard comparator group, mean percentage of Hematocrit value at screening was 43.05 ± 5.74 which slightly reduced to 42.79 ± 4.08 at Day 1, 41.79 ± 4.1 at Day 2, 41.11 ± 4.4 at Day 3, 39.95 ± 3.47 at Day 4, further reduced to 38.95 ± 3.87 (p<0.0231) at Day 6 of compared to that of baseline, 38.89 ± 4.12 (p<0.0308) at Day 8 compared to that of baseline and 38.84 ± 4.18 (p<0.0287) at Day 10 compared to that of the baseline.
In Platenza group, mean duration of Prothrombin Time (seconds) at Screening was 11.71 ± 1.59 which slightly reduced to 11.62 ± 1.24 at Day 1, further reduced to 11.43 ± 1.21 at Day 2, 11.86 ± 1.06 at Day 3, 12.19 ± 1.08 at Day 4, 12.19 ± 1.03 at Day 6, 12.48 ± 1.08 at Day 8 and 12 ± 1.04 at Day 10. Although there was a trend of increase in the Prothrombin Time (seconds), all the values were within the normal range and the results were statistically insignificant compared to neither the baseline nor the Standard comparator group. In Standard comparator group, mean duration of Prothrombin Time (seconds) at screening was 11.16 ± 1.17 which slightly increased to 11.47 ± 1.26 at Day 1, further increased to 11.63 ± 1.17 at Day 2, 11.84 ± 1.02 (p<0.0159) at Day 3 compared to that of the baseline, 12.05 ± 0.91 at Day 4, but reduced to 11.89 ± 0.94 at Day 6, once again increased to 12.26 ± 0.73 (p<0.0069) at Day 8 compared to that of the baseline and reduced to 11.97 ± 0.72 at Day 10. At Day 10, although there was a trend of decrease in the Prothrombin Time (seconds), all the values were within the normal range and the results were statistically insignificant.
In Platenza group, mean duration of Clotting Time (minutes) at Screening was 3.61 ± 0.94 slightly increased to 3.69 ± 0.88, 3.83 ± 0.67, 3.96 ± 0.62, 3.67 ± 0.69, 3.69 ± 0.75, 3.6 ± 0.72, 3.65 ± 0.72 at Day 1, Day 2, Day 3, Day 4, Day 6, Day 8 and Day 10 respectively. At Day 10, although there was a trend of increase in the Clotting Time (minutes), all the values were within the normal range and the results were statistically insignificant. In Standard comparator group, mean duration of Clotting Time (minutes) at screening and Day 1 was 3.94 ± 1.25, 3.91 ± 1.17 which slightly reduced to 3.85 ± 1.09, 3.83 ± 1.1, 3.83 ± 1.15 at Day 2, Day 3 and Day 4, but slightly increased to 3.93 ± 1, 4 ± 0.95, 4.1 ± 0.93 at Day 6, Day 8 and Day 10 respectively. At Day 10, although there was a trend of increase in the Clotting Time (minutes) since day 8, all the values were within the normal range and the results were statistically insignificant.
In Platenza group, mean duration of Bleeding Time (minutes) at Screening and Day 1 was 2.16 ± 1.01, 2.16 ± 1.01 which slightly reduced to 2.14 ± 1.02, 2.09 ± 0.95 at Day 2 and Day 3, and gradually increased to 2.16 ± 1.06, 2.17 ± 1.02, 2.18 ± 1.01, 2.17 ± 1.02 at Day 4, Day 6, Day 8 and Day 10 was respectively. Although at Day 4, Day 6, Day 8 and Day 10, there was a trend of increase in the Bleeding Time (minutes), all the values were within the normal range and the results were statistically insignificant. In Standard comparator group, mean duration of Bleeding Time (minutes) at screening was 2.39 ± 1.04 which slightly reduced to 2.21 ± 0.98, 2.21 ± 0.84 at Day 1 and Day 2 and gradually increased to 2.24 ± 0.97, 2.34 ± 0.95, 2.41 ± 1.03, 2.44 ± 1.01, 2.41 ± 1.03 at Day 3, Day 4, Day 6, Day 8 and Day 10 respectively. Although at Day 3, Day 4, Day 6, Day 8 and Day 10, there was a trend of increase in the Bleeding Time (minutes), all the values were within the normal range and the results were statistically insignificant compared to neither the baseline nor the Standard comparator group.
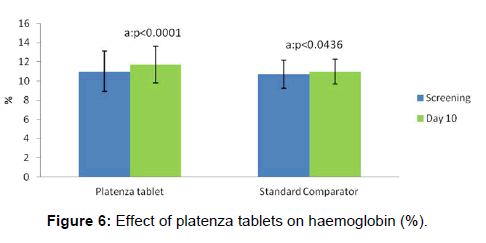
The efficacy of platenza tablets on hemoglobin (%) is graphically represented in Figure 6. In Platenza group, mean percentage of hemoglobin count at Screening was 11 ± 2.1 which slightly increased to 11.71 ± 1.89 (p<0.0001) at Day 10 compared to that of baseline. In Standard comparator group, mean percentage of hemoglobin count at screening was 10.71 ± 1.47 which slightly increased to 10.99 ± 1.31 (p<0.0436) at Day 10 compared to that of the baseline [Table 4].
In Platenza group, mean value of total WBC count at Screening was 7143 ± 815.2 which slightly increased to 7181 ± 696.1 at Day 10. At Day 10, although there was trend of increase in total WBC count compared to baseline, all the values were within normal range and the results were statistically insignificant. In Standard comparator group, mean value of total WBC count at screening was 7547 ± 1016 which slightly reduced to 7226 ± 629.7 at Day 10. At Day 10, although there was trend of decrease in total WBC count compared to baseline, all the values were within normal range and the results were statistically in significant.
In Platenza group, mean value of Neutrophil count at screening was 70 ± 3.55 which reduced to 66.86 ± 3.53 (p<0.0005) at Day 10 compared to that of the baseline. In Standard comparator group, mean value of Neutrophil count at screening was 70.26 ± 5.02 which reduced to 66.89 ± 5.36 (p<0.0011) at Day 10 compared to that of the baseline.
In Platenza group, mean value of lymphocyte count at screening was 25.9 ± 4.05 which slightly increased to 28.71 ± 3.72 (p<0.0024) at Day 10 compared to that of the baseline. In Standard comparator group, mean value of lymphocyte count at screening was 25.63 ± 4.15 which slightly increased to 28.26 ± 3.63 (p<0.0016) at Day 10 compared to that of the baseline.
In Platenza group, mean value of eosinophil count at Screening was 2.52 ± 1.08 which slightly increased to 2.57 ± 1.17 at Day 10. At Day 10, although there was a trend of increase in Eosinophil count compared to baseline, all the values were within normal range and the results were statistically insignificant. In Standard comparator group, mean value of eosinophil count at screening was 2.58 ± 1.54 which almost remained same at 2.58 ± 1.74 at Day 10. At Day 10, although there was a trend of no change in eosinophil count compared to baseline, all the values were within normal range and the results were statistically insignificant.
In Platenza group, mean value of monocyte count at screening was 1.43 ± 0.93 which slightly increased to 1.48 ± 0.87 at Day 10. At Day 10, although there was a trend of increase in monocyte count compared to baseline, all the values were within normal range and the results were statistically insignificant. In Standard comparator group, mean value of monocyte count at screening was 1.63 ± 0.76 which increased to 1.95 ± 0.78 at Day 10. At Day 10, although there was a trend of increase in monocyte count compared to baseline, all the values were within normal range and the results were statistically insignificant.
In Platenza group, mean value of basophil count at screening was 0.76 ± 0.77 which slightly increased to 1.1 ± 0.77 at Day 10. At Day 10, although there was a trend of increase in basophil count compared to baseline, all the values were within normal range and the results were statistically insignificant. In Standard comparator group, mean value of basophil count at screening was 0.83 ± 0.92 which slightly increased to 1.22 ± 1.22 at Day 10. At Day 10, although there was a trend of increase in basophil count compared to baseline, all the values were within normal range and the results were statistically insignificant.
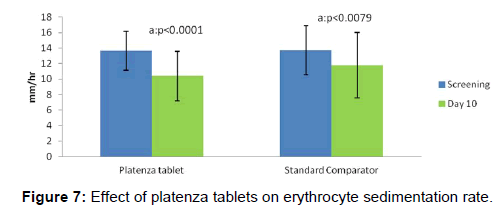
The efficacy of platenza tablets on erythrocyte sedimentation rate is graphically represented in Figure 7. In Platenza group, mean value of erythrocyte sedimentation rate at screening was 13.62 ± 2.5 which reduced to 10.38 ± 3.22 (p<0.0001) at Day 10 compared to that of the baseline. In Standard comparator group, mean value of erythrocyte sedimentation rate at screening was 13.68 ± 3.15 which slightly reduced to 11.79 ± 4.2 (p<0.0079) at Day 10 compared to that of the baseline.
In Platenza group, mean value of serum glutamic-pyruvic transaminase at screening was 32.62 ± 9 which reduced to 27.52 ± 10.4 (p<0.0211) at Day 10 compared to that of the baseline. In Standard comparator group, mean value of serum glutamicpyruvic transaminase at screening was 35.67 ± 8.11 which reduced to 28.11 ± 8.5 (p<0.007) at Day 10 compared to that of the baseline.
In Platenza group, mean value of serum creatinine at screening was 0.78 ± 0.13 which reduced to 0.66 ± 0.09 (p<0.0013) at Day 10 compared to that of the baseline. In Standard comparator group, mean value of serum creatinine at screening was 0.79 ± 0.13 which reduced to 0.7 ± 0.11 (p<0.0083) at Day 10 compared to that of the baseline.
In Platenza group, there was a significant improvement in the quality of life related to the fatigue, sense of feeling week, sense of dizziness, sense of feeling sad or depressed, sense of feeling cold in hand and feet compared to the baseline and Standard comparator group [Table 5].
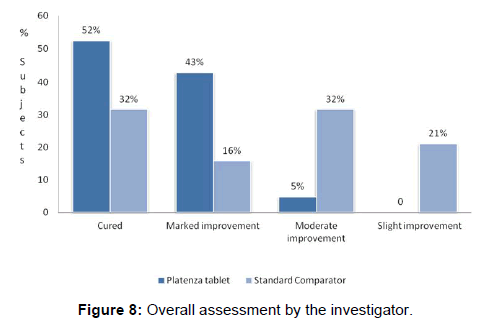
In Platenza group, out of 21 subjects, 11 subjects showed complete cure accounting to about 52%; further 9 subjects out of 21 subjects showed marked improvement accounting to about 43%; remaining 1 subject out of 21 subjects showed moderate improvement accounting to 5% [Figure 8 and Table 6].
In Standard comparator group, out of 19 subjects, 06 subjects showed complete cure accounting to about 32%; further 03 subjects out of 19 subjects showed marked improvement accounting to about 16%; only 06 subjects out of 19 subjects showed moderate improvement accounting to 32%, remaining 04 subjects out of 19 subjects showed slight improvement accounting to 21%.
Safety Analysis
The hematological and biochemical parameters for safety evaluation were within the normal limits in both the groups. In platenza group, only 1 subject presented with mild nausea and 2 subjects presented with mild abdominal discomfort and in standard comparator group, 2 subjects presented with mild abdominal discomfort. However; in both the groups, these events did not necessitate treatment discontinuation and the above mentioned mild symptoms subsided with continued treatment.
Discussion
During this study, significant reduction in the mean score for clinical symptoms was observed. The platelet count was significantly normalized by the end of the study in platenza group as compared to the standard comparator group. All the hematological and biochemical parameters were within the normal range with significant reduction in Serum Glutamic Pyruvic Transaminase levels in both platenza group and standard comparator group before and after treatment.
There was a significant improvement in the quality of life related to the fatigue, sense of feeling week, sense of dizziness, sense of feeling sad or depressed, sense of feeling cold in hand and feet compared to that of baseline and standard comparator group. The overall response of the platenza group showed 52% of the total subjects completely free from dengue symptom compared to 32% in the standard comparator group and good subject compliance was observed. There were no clinically significant adverse reactions/events neither observed nor reported during the study period.
Potent Ayurvedic herbs like Carica papaya [Eranda Karkati] known to for its anti-dengue and platelet augmentation activity and reported to be having enhancement of arachidonate 12‑lipoxygenase and the platelet activating factor receptor gene expression which is responsible for increased platelet production. It is also known to decrease peripheral platelet destruction by membrane stabilizing activity due to presence of flavonoids and other phenolic compounds might have potentially facilitated the significant increase in platelet counts [1]. Tinospora cordifolia [Guduchi] which is known to strengthen host immune system by activating macrophages, NF-kappa beta translocation and cytokine production in addition its anti-pyretic, antiviral and hepato-protective activity [7] along with Phyllanthus madraspatensis [Bhudhatri] which is reputed for its anti-viral and hepatoprotective activity [4] and finally with the potent Antipyretic activity [5] and bioavailability enhancing properties [6] of piperine from Piper nigrum might have potentially facilitated the significant reduction in clinical signs and symptoms of dengue fever associated with thrombocytopenia.
Thus, the proposed poly herbal preparation “Platenza tablet” is hypothetically expected to reverse the low platelet counts due to dengue and other clinical conditions which are associated with mild to moderate thrombocytopenia thus hastens the faster recovery.
Based on the above results mentioned; In Platenza group, there was a statistically significant improvement in hemoglobin percentage, total WBC counts, Neutrophil count at the end of the study (Day 10) suggesting the safety of platenza tablets at the recommended dose and statistically significant improvement in the clinical parameters along with the study specific efficacy parameter at the end of the study (Day 10) suggesting the potential and enhanced efficacy of platenza tablets at the recommended dose compared to that of Standard comparator.
Conclusion
In conclusion; from the result of the present study, platenza tablet is potentially safe and efficacious in normalizing the platelet counts and relieving the clinical signs and symptoms (fever, myalgia, arthralgia, headache) of dengue fever associated with thrombocytopenia than compared to that of the standard comparator consisting of Carica papaya leaf extracts alone.
It is also conclusively indicative that platenza tablets should be used as an additional or as a complementary drug in patients with dengue fever to relieve the signs and symptoms of dengue fever associated with thrombocytopenia and platenza tablets accelerate the increase in platelet counts thereby shortening the period of hospitalization.
Conflict of Interest
All authors disclose that there was no conflict of interest.
REFERENCES
- Sarala N, Paknikar. Papaya extract to treat dengue: A novel therapeutic option? Annals of Medical and Health Sciences Research 2014;4:32-24.
- Dengue- Disease management and investigative guidelines, Kansan Department of health and environment, division of health 2015.
- Xu M, Zuest R, Velumani S, Tukijan F, Toh YX, Appanna R, et al. A potent neutralizing antibody with therapeutic potential against all four serotypes of dengue virus. NPJ Vaccines. 2017;2:2.
- Sharma SK, Arogya SM, Bhaskarmurthy DH, Agarwal A, Velusami CC. Hepatoprotective activity of the Phyllanthus species on tert-butyl hydroperoxide (t-BH)-induced cytotoxicity in HepG2 cells. Pharmacognosy magazine. 2011;7:229.
- Sabina EP, Nasreen A, Vedi M, Rasool M. Analgesic, antipyretic and ulcerogenic effects of piperine: an active ingredient of pepper. Journal of Pharmaceutical Sciences and Research. 2013;5:203-206.
- Wadhwa S, Singhal S, Rawal S. Bioavailability enhancement by piperine: A review. Asian Journal of Biomedical and Pharmaceutical Sciences. 2014;4:1.
- Kavitha BT, Shruthi SD, Rai SP, Ramachandra YL. Phytochemical analysis and hepatoprotective properties of Tinospora cordifolia against carbon tetrachloride-induced hepatic damage in rats. Journal of Basic and Clinical Pharmacy. 2011;2:139.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.