Clinical Profile, Maternal and Fetal Outcomes of Acute Hepatitis E in Pregnancy
- *Corresponding Author:
- Dr. Tushar B Patil
Plot No. 9, Rashtrasant Nagar, Godhani Road, Zingabai Takli, Nagpur - 440 030, Maharashtra, India.
E-mail: dr.tushar42@rediffmail.com
Abstract
Background: Pregnant women are at increased risk of complications in hepatitis E virus (HEV) infection, with the risk increasing as the pregnancy progresses, often leading to fulminant hepatic failure and adverse fetal outcome. Aims: The primary objective of the following study is to evaluate the maternal and fetal complications of this infection and secondary aim is to compare the clinical features of hepatitis E in pregnant women to those in non‑pregnant women. Subjects and Methods: This was a hospital based case‑controls study, carried out from July 2008 to June 2010. Over a period of 2 years, cases were serologically confirmed pregnant women with hepatitis E, selected by screening in antenatal clinic. Controls were serologically confirmed non‑pregnant women with hepatitis E, selected by screening in Medicine Outpatient Department. We studied 96 women with HEV infection, of which 52 were pregnant and 44 were non‑pregnant. Clinical and laboratory profile of patients in both groups were studied. Patients were treated as per protocol and the outcome was studied in both groups. Pregnant women were followed‑up for fetal and maternal outcome. We used t‑test and z‑test to compare normally distributed data and non‑normally distributed data, respectively. Chi‑square test was used to compare discrete values between groups. Results:Mean (standard deviation [SD]) age in pregnant patients was 24.1 (3.3) years while 32.6 (10.5) years in non‑pregnant patients. 71.1% (37/52) of the patients were primigravida and 28.8% (15/52) patients were multigravida, by natural occurrence. Mean (SD) gestational age when infection occurred was 27.5 (7.2) weeks. Among pregnant women, 63.4% (33/52) were in 3rd trimester. Jaundice 1‑5 days before presentation was seen in 51.9% (27/52) pregnant and 44.2% (23/44) non‑pregnant women. Myalgia/arthralgia, fever, nausea/vomiting, right upper quadrant pain, jaundice, dark urine, light‑colored stools, pruritus, diarrhea, altered sensorium and hematemesis/melena were presenting features. In pregnant group, 46.1% (24/52) patients developed encephalopathy while in non‑pregnant group 34% (15/44) developed this complication. Among pregnant cases, 67.3% (35/52) survived and 32% (17/52) cases died. In non‑pregnant group, nearly 90% (40/44) patients survived and only 9% (4/44) patients died. This difference was statistically significant (P < 0.01). Adverse fetal outcome was seen in 71.1% (37/52) pregnant women with acute hepatitis E, including pre‑term delivery in 23% (12/52), stillbirth in 23% (12/52), abortion in 3.8% (2/52) and intra‑uterine fetal death in 21.1% (11/52) patients. Conclusions: There is significantly higher occurrence of hepatitis E infection in pregnant women than in non‑pregnant women, which increases with gestation, with associated fulminant hepatic failure, maternal mortality and worse fetal outcome.
Keywords
Fetal outcome, Hepatitis E, Jaundice, Pregnancy
Introduction
Hepatitis E is an acute viral infection caused by hepatitis E virus (HEV), which is a 32-34 nm, non-enveloped virus with a 7600-nucleotide, single-strand, positive-sense ribonucleic acid genome. It is endemic in India, Asia, Africa, the Middle East and Central America. This agent is the most common cause of acute viral hepatitis in the adult population in India. The first retrospectively described outbreak of hepatitis E occurred in India in 1955-1956.[1] The main source of transmission of HEV is contaminated drinking water. Acute infection is generally mild and self-limited, having incubation period of 8-10 weeks with a clinical illness resembling other forms of acute viral hepatitis and resolving within 6 weeks, with no chronic sequelae. Fulminant hepatitis due to acute hepatitis E occurs rarely in adult men and non-pregnant women with a low case-fatality rate (<0.1%).
Pregnant women are at increased risk of complications in HEV infection, with the risk increasing as the pregnancy progresses, often leading to fulminant hepatic failure and death in a high number of cases. Acute HEV infection is especially severe during second and 3rd trimester of pregnancy and it may lead to fulminant hepatic failure and death in 30-100% of patients.[2] There is also increased number of obstetric complications, e.g., premature rupture of membranes, postpartum hemorrhage, spontaneous abortions, intra-uterine fetal death etc., The fetal complications include prematurity and low birth weight. In a study from Bangladesh, it was observed that 19% to 25% of all maternal deaths and 7-13% of all neonatal deaths were associated with jaundice in pregnant women. Further, 58% of deaths in pregnant women with acute liver disease in hospitals were associated with HEV.[3]
In pregnant women, HEV infection is more severe, Information is limited and conflicting on the effect of HEV on maternal, obstetric and fetal outcomes. Hence, this study was undertaken to study clinical profile of hepatitis E infection in pregnancy, to evaluate the maternal and fetal complications of this infection and to compare the clinical features of hepatitis E in pregnant women to those in non-pregnant women.
Subjects and Methods
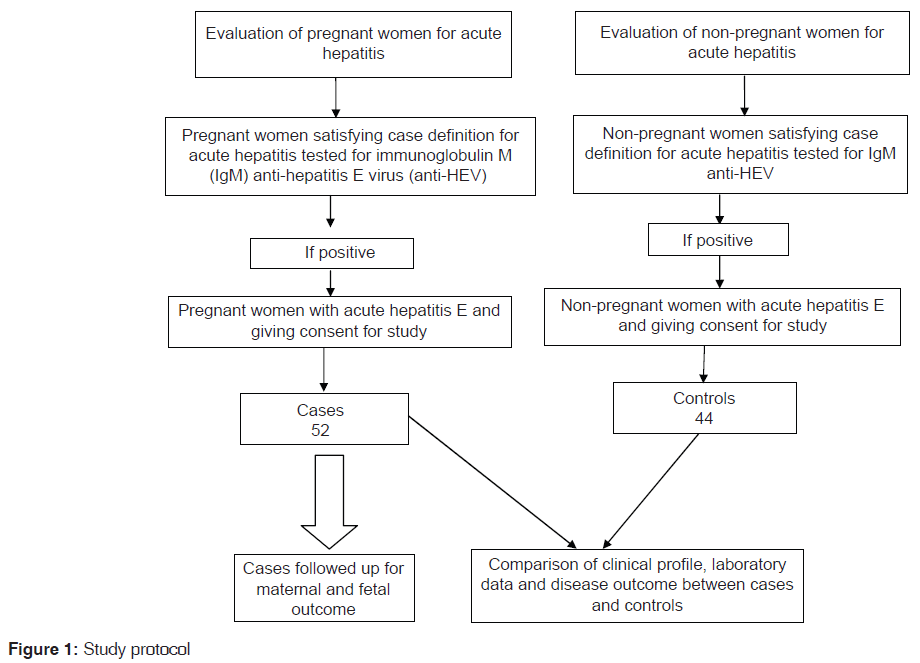
This was a hospital based case control study to compare the disease characteristics and outcomes of hepatitis E between pregnant and non-pregnant women. The study was carried out in a tertiary care teaching hospital in Central India from July 2008 to June 2010. The study was started after obtaining approval of the Institutional Ethics Committee. Patients were enrolled in the study after obtaining written informed consent. Study methodology is shown in Figure 1.
Selection of cases
All pregnant women visiting antenatal clinic of our hospital were screened for acute hepatitis. The diagnosis of acute hepatitis was made according to the World Health Organization (WHO) case definition for acute hepatitis, which defines acute hepatitis as “an acute illness typically including acute jaundice, dark urine, anorexia, malaise, extreme fatigue and right upper quadrant tenderness. Biological signs include increased urine urobilonogen and >2.5 times the upper limit of serum alanine aminotransferase.” As per WHO protocol, these patients were subjected to serological examination for viral hepatitis with enzyme-linked immunosorbent assay (ELISA) for immunoglobulin M (IgM) anti-hepatitis A virus (HAV) antibodies, hepatitis B surface antigen (HBsAg), anti-hepatitis C virus antibodies (anti-HCV) and IgM anti-HEV (IgM anti-HEV) antibodies. A confirmed case of hepatitis E was defined as a patient who satisfied the WHO case definition for acute hepatitis and was positive for IgM anti-HEV antibodies.
Exclusion criteria
Patients with hepatitis other than hepatitis E, dual viral infection, drug induced hepatitis, cholestatic jaundice of pregnancy/acute fatty liver of pregnancy, congestive hepatopathy, hepatitis due to severe infection or multiorgan failure, hemolysis, elevated liver enzymes, low platelets syndrome, clinical or laboratory evidence of chronic liver disease, alcoholic liver disease and hemolytic jaundice were excluded from the study.
After applying above inclusion and exclusion criteria, 84 pregnant women satisfied the definition for a confirmed case of hepatitis E. However, 32 patients refused to participate in the study and were excluded. Remaining 52 patients were included as “cases” after obtaining a written informed consent.
Selection of controls
Women visiting our Medicine Outpatient Department were screened for features of acute hepatitis and those who satisfied the WHO case definition for acute hepatitis were subjected to serological examination with ELISA for IgM anti-HAV, HBsAg, anti-HCV and IgM anti-HEV antibodies. Women with acute hepatitis and patients positive for IgM anti-HEV antibodies were evaluated for exclusion criteria as above. During the study period, a total of 68 non-pregnant women satisfied definition for a confirmed case of hepatitis E. However, consent for participation in the study could be obtained in only 44 patients, who were included as controls. The selection of control was done on a random basis.
Detailed history taking and clinical examination was performed in cases and controls. Investigation included complete blood counts, liver function test, kidney function test, serum electrolytes and ultrasonography of the abdomen and examination of ascetic fluid, when present. Duration of gestation was estimated from duration of amenorrhea and pelvic ultrasound findings. Clinical and laboratory data were compared between the two groups. All the patients were followed-up to their hospital stay. Pregnant women were followed-up to know their obstetric outcome.
Management depended on whether the patient’s acute viral hepatitis was complicated by fulminant hepatic failure, which was diagnosed when hepatic encephalopathy developed in a patient with acute viral hepatitis within 8 weeks of the onset of jaundice.[4] Patients without fulminant hepatic failure were given standard care and were monitored for signs and complications of acute viral hepatitis (fever, edema, ascites, paralytic ileus, nasal and gastrointestinal (GI) bleeding, high leukocyte count, high creatinine concentration, hepatic encephalopathy, clinically significant coagulation defect, hypoglycemia, hyponatremia, hypernatremia, hypokalemia, hyperkalemia and hypocalcemia) and obstetric complications (antepartum, intrapartum, or postpartum hemorrhage; premature rupture of membranes; and intra-uterine death). If their condition improved, patients were discharged and advised to return for regular outpatient follow-up visits until delivery.
Patients with fulminant hepatic failure were managed with supportive care in the intensive care unit. They were monitored for medical and obstetric complications. They received 20% mannitol, lactulose, antibiotics, parenteral nutrition and ventilatory support, as needed. All women with manifestations of bleeding were infused with fresh frozen plasma and packed red cells.
Statistical analysis
We used the t-test and the z-test to compare normally distributed data and non-normally distributed data, respectively, of HEV-infected pregnant and non-pregnant patients. The Chi-square test was used to compare discrete values between groups. A (P < 0.05) was considered to be significant. Statistical analyses were performed by using Statistical Package for the Social Sciences software version 16.0 (Chicago IL, USA).
Results
During the study period of 2 years, 2140 deliveries were conducted in our hospital. Out of these 2140 pregnant women, 84 women had acute hepatitis E infection. Thus, the overall prevalence of hepatitis E in pregnancy was observed to be 3.9% (84/2140). However, 32 women did not give consent for the study and were excluded. Remaining 52 patients were included and studied in detail.
Age distribution
We studied 96 women with acute HEV infection, of which 52 were pregnant and 44 were non-pregnant. Mean (standard deviation [SD]) age in pregnant patients was 24.1 (3.3) years while 32.6 (10.5) years in non-pregnant patients. Half of pregnant women who had infection were in the age group of 23-27 years; the next most common age group was between 18 and 22 years, who constituted 38.4% (20/52) of pregnant women. Thus, 88.4% (46/52) of pregnant women with acute HEV infection were in the age group of 18-27 years. However, in non-pregnant group the infection incidence was moreover evenly spread. Highest percentage, i.e., 22.7% (10/44) of non-pregnant cases was seen in the age group of 23-27 years.
Gravida status and gestational age
Among the pregnant women, 71.1% (37/52) patients were primigravida and 28.8% (15/52) patients were multigravida. Mean (SD) gestational age when infection occurred was 27.5 (7.2) weeks. It was seen that 63.4% (33/52) patients were in 3rd trimester of pregnancy. Total 32.6% (17/52) of cases were in 2nd trimester and only 3.8% (2/52) developed an infection in 1st trimester.
Clinical presentation
Table 1 shows the duration of jaundice in patients before admission and Table 2 shows predominant presenting clinical symptoms. In pregnant group, 46.1% (24/52) patients developed encephalopathy while in non-pregnant group 34% (15/44) developed this complication. Among pregnant women, 28.8% (15/52) had jaundice to encephalopathy interval of 1-3 days. Similarly, 25% (11/44) patients in non-pregnant group had jaundice encephalopathy duration of 1-3 days. No patients from either of group had jaundice to encephalopathy interval >9 days. Laboratory parameters in the patients are shown in Table 3.
| Duration of jaundice before admission (in days) | Pregnant | Non-pregnant | Chi- square value | P value | ||
|---|---|---|---|---|---|---|
| (n=52) | % | (n=44) | % | |||
| 1-5 | 27 | 51.9 | 23 | 44.2 | 2.69 | 0.60 |
| 6-10 | 19 | 36.5 | 18 | 34.6 | ||
| 11-15 | 3 | 5.8 | 3 | 5.8 | ||
| 16-20 | 2 | 3.8 | 00 | 0 | ||
| No jaundice | 1 | 1.9 | 00 | 0 | ||
| Total | 52 | 100 | 44 | 100 | ||
Table 1: Duration of jaundice in patients with hepatitis E (n=6)
| Symptoms | Pregnant | Non-pregnant | Z value | ||
|---|---|---|---|---|---|
| (n=52) | % | (n=44) | % | ||
| Myalgia/arthralgia | 28 | 53.8 | 32 | 72.7 | 1.86, NS |
| Fever | 12 | 21.1 | 25 | 56.8 | 3.82, S |
| Nausea/vomiting | 52 | 100 | 41 | 93.1 | 1.81, NS |
| Right upper quadrant | 18 | 34.6 | 10 | 22.7 | 1.20, NS |
| pain | |||||
| Jaundice | 45 | 86.5 | 43 | 97.7 | 1.87, NS |
| Dark urine | 35 | 67.3 | 31 | 70.4 | 0.31, NS |
| Light-colored stools | 4 | 7.6 | 1 | 2.2 | 1.21, NS |
| Pruritus | 6 | 11.5 | 7 | 15.9 | 0.57, NS |
| Diarrhea | 3 | 5.7 | 0 | 00 | 1.65, NS |
| Altered sensorium | 11 | 21.1 | 7 | 15.9 | 0.49, NS |
| Hematemesis/melena | 1 | 1.9 | 1 | 2.2 | 0.31, NS |
S: Significant, NS: Non-significant, Z-tabulated value=1.96
Table 2: Predominant presenting clinical symptoms (n=96)
| Parameter | Pregnant mean(SD) value (n=52) | Non-pregnant mean (SD) value (n=44) | Z value | P value |
|---|---|---|---|---|
| Hemoglobin | 9.3(1.7) | 9.1(2.7) | 0.56 | 0.57 |
| in (g/dl) | ||||
| TLC (/mm3) | 13131.6 (6864.1) | 8200 (3461.9) | 4.54 | <0.001 |
| Platelete | 210.4 (92.9) | 181.4 (73.5) | 1.7 | 0.09 |
| count (lacs) | ||||
| Serum | 1.2(0.7) | 1.4(0.7) | 1.87 | 0.06 |
| creatinine | ||||
| (mg/dl) | ||||
| Total | 5.3(0.7) | 5.6(0.8) | 1.62 | 0.10 |
| proteins | ||||
| (g/dl) | ||||
| Serum | 11.9 (4.9) | 9.2(5.1) | 2.61 | 0.01 |
| bilirubin | ||||
| (mg/dl) | ||||
| SGOT | 510.4(711.3) | 129.9(166.9) | 3.74 | <0.001 |
| (IU/L) | ||||
| SGPT | 485.3(586.5) | 152.6(224.6) | 3.78 | <0.001 |
| (IU/L) | ||||
| Prothrombin | 13.3(19.9) | 9.5 (14) | 1.07 | 0.28 |
| time (s) |
SD: Standard deviation, TLC: Total leucocyte count, SGOT: Serum glutamic-oxaloacetic transaminase, SGPT: Serum glutamic pyruvic transaminase
Table 3: Laboratory parameters (n=96)
Mean (SD) serum bilirubin level was 11.9 mg/dl (4.9) in pregnant and 9.2 mg/dl (5.1) in non-pregnant patients. In pregnant women, 38.4% (20/52) patients had serum bilirubin levels between 11 and 15 mg/dl and 36.5% (19/52) of patients had serum bilirubin between 6 and 10 mg/dl. Bilirubin levels between 16 and 25 mg/dl were seen in 19.2% (10/52) of pregnant women. Only 3.8% (2/52) of patients developed jaundice <5 mg/dl. In non-pregnant group 25% (11/44) patients had bilirubin level up to 5 mg/dl. Majority of patients i.e., 47.7% (21/44) had bilirubin level of 6-10 mg/dl. Non-pregnant patients had milder jaundice, i.e. bilirubin in the range of 1-10 mg/dl while pregnant patients had deeper jaundice between 6 and 15 mg/dl of range and this difference was statistically significant (z - 2.69). Among pregnant women, 53.8% (28/52) had acute viral hepatitis while 46.1% (24/52) of patients had presented with acute hepatic failure. Among non-pregnant group, 29/44 (65.9%) of patients presented with acute viral hepatitis while 34% (15/44) patients had acute liver failure on admission. Medical complications in the patients are depicted in Table 4. Among pregnant women, 67.3% (35/52) patients survived and 32.6% (17/52) patients died. In non-pregnant group, 90.9% (40/44) patients survived and only 9% (4/44) patients died. This difference was statistically significant (P < 0.01). Table 5 shows fetal outcome in pregnant women with hepatitis E.
| Complications | Pregnant | Non-pregnant | Z value | ||
|---|---|---|---|---|---|
| (n=52) | % | (n=44) | % | ||
| Ascites | 14 | 26 | 5 | 11 | 1.94, NS |
| Coagulopathy | 22 | 42 | 24 | 54 | 1.18, NS |
| GI bleed | 13 | 25 | 16 | 36 | 1.16, NS |
| Renal failure | 11 | 21 | 7 | 15 | 0.76, NS |
| Seizures | 3 | 5 | 5 | 11 | 1.07, NS |
| Encephalopathy | 24 | 46 | 15 | 34 | 1.20, NS |
NS: Non-significant, GI: Gastrointestinal
Table 4: Medical complications
| Outcome | No. (%) |
|---|---|
| Full-term | 15 (28.8) |
| Pre-term | 12 (23.1) |
| Stillbirth | 12 (23.1) |
| Abortion | 2 (3.8) |
| Intra-uterine fetal death | 11 (21.1) |
Table 5: Fetal outcome in pregnant patients with hepatitis E (n=52)
Discussion
HEV infection has complex and not yet completely clarified, clinical-epidemiological characteristics. HEV-infected pregnant women have a higher rate of fulminant hepatic failure and higher mortality rate, compared with HEV-infected non-pregnant women. Women with HEV infection were also more likely to have obstetric complications, such as antepartum hemorrhage and intra-uterine fetal death and poor fetal outcome, including preterm delivery and stillbirth. There are many studies from Indian and other Asian authors embarking upon the presentation and mortality of this infection in affected people. This study however compares two groups, i.e., one is pregnant and other is non-pregnant in terms of incidence, disease characteristics, type of presentation and overall outcome. Clinical infection concentrates among adolescents and young adults in countries of high endemicity. During outbreaks, the clinical attack rate (3-30%) is highest among pregnant women.[5]
Gravida status and gestational age
We observed that 71% patients were primigravida and 29% patients were multigravida. Median gestational age was 27.5 weeks and 63% cases were in 3rd trimester of pregnancy. Nearly 32% of cases were in 2nd trimester and only 5% developed an infection in 1st trimester. This observation is similar to previous studies, which showed increasing incidence as duration of gestation increases. The relation between gravid status and incidence was not studied in previous studies. However, our study showed significantly higher incidence of disease in primigravida population.
The high mortality rate of acute hepatitis E in pregnancy is postulated to result from associated hormonal (estrogen and progesterone) and immunological changes including downregulation of nuclear factor-kappa-B and shift in T helper-1 cells/T helper-2 cells (Th2) balance toward Th2 along with host susceptibility factors which occur in pregnant state.[6-8] Devhare et al. studied the immune response to HEV infection and observed early cellular response in HEV infection and associated molecular mechanisms suggesting the potential role of the inflammatory response triggered by HEV infection in host immune response and pathogenesis. They demonstrated up regulation of cytokine and chemokine genes and secretion of interleukin-6 (sIL-6), IL-8 and tumor necrosis factor-α by human epithelial cells infected with HEV.[9] Tripathy et al. found that elevated IL-1 α and sIL-2 receptor α (sIL-2R α) levels in the blood are pivotal in the pathogenesis of HEV. They also demonstrated involvement of innate immune response at the site of infection.[10] Furthermore, Srivastava et al. suggested that interferon-γ secreting CD4 lymphocytes are involved in immune response and are related to intrahepatic sequestration of immune response.[11] All these studies suggest that the immune mediated destruction of hepatocytes is important in the pathogenesis of hepatitis E.
Premature deliveries with high infant mortality of up to 33% are also observed. In pregnant women in 3rd trimester with viral hepatitis, the prevalence of HEV infection is reportedly between 40% and 57%.[12] Frequency of acute HEV infection and mortality increases with the gestational age.[13] In a previous study,[13] it was seen that two patients (4.8%) were in the 1st trimester of pregnancy, 14 (33.3%) in the 2nd trimester, and 26 (61.9%) in the 3rd trimester; whereas 12 (28.6%) women were primigravida. Mean gestational age was 31 weeks. 33% patients were affected in 2nd trimester and 67% patients in 3rd trimester in another study.[8]
Duration of jaundice at the time of admission
Out of all pregnant females, 53% had jaundice of <5 days before admission, 36.4% of patients had jaundice of duration 6-10 days; 5.8% had jaundice of 11-15 days and 3.8% patients had jaundice of 16-20 days. Mean duration of jaundice in this group was 5 days. Out of all non-pregnant patients, 44.2% had jaundice of <5 days duration, 34.6% had jaundice of 6-10 days, and 5.8% had jaundice of 11-15 days duration. Mean duration of jaundice in this group was 4 days. There was no significant difference between two groups as far as duration of jaundice is considered. Asymptomatic or subclinical cases are described in non-pregnant population. There was one pregnant patient who was unicteric. This presentation is rare and unusual; not described previously.
Median duration of jaundice before admission was 4 days in pregnant patients, as observed by Patra et al.[14] A comparative analysis has shown that the mean duration of jaundice before admission in pregnant patients who survived was 8 days and 10 days in patients who didn’t survive.[13]
Predominant presenting clinical symptoms
In most of the men and non-pregnant women, the infection with HEV is self-limiting, with the average incubation period being 40 days; and patient develop moderate jaundice, and complete resolution of hyperbilirubinemia and normalization of aminotransferases within 1-6 weeks.[15] However, the severity of the disease may range from mild to fulminant in pregnant women. It was seen that nausea/vomiting (100% vs. 93%) and jaundice (87% vs. 97%) were most predominant symptoms in both the groups respectively. Fever was significantly more common (58% vs. 22%) in non-pregnant patients as the predominant presentation than in pregnancy (z - 3.82), the other symptoms present were dark urine, myelgia/artharlgia, right upper quadrant pain, fever, altered sensorium, pruritus light-colored stools, diarrhea, and hematemesis/malena. Occurrence of pruritus, myalgia/arthralgia and jaundice was more common in non-pregnant group but was statistically not significant.
Jaundice to encephalopathy interval
We compared the jaundice to encephalopathy interval in both groups. More pregnant patients develop encephalopathy than non-pregnant but this difference was statistically not significant. It has been observed that a longer jaundice-to-encephalopathy duration is associated with a worse clinical outcome in acute hepatic failure.[16] Jaundice to encephalopathy interval in patients who were pregnant and developed acute liver failure was observed and found to be 7 days in both groups who survived and who didn’t.[13]
Laboratory parameters
Pregnant patients had higher leukocyte count on admission and also liver enzymes were elevated as compared to non-pregnant group. Jaundice <5 mg/dl was seen in only 3% of pregnant women. However in non-pregnant group, 25% patients had bilirubin level upto 5 mg/dl. It was further seen that 38.5% of pregnant patients had serum bilirubin levels between 11 and 15 mg/dl and 36.5% had bilirubin between 6 and 10 mg/dl. Mean serum bilirubin level was 11.9 mg/dl in pregnancy but 9.2 mg/dl in non-pregnant patients. Thus non-pregnant patients tend to develop milder jaundice i.e., bilirubin in the range of 1-10 mg/dl. While pregnant patients tend to develop deeper jaundice between 06 and 15 mg/dl of range and this difference is statistically significant. HEV infected patients with severe jaundice had significantly lower peak serum levels of γ-glutamyl-transpeptidase, lower albumin levels, lower acetylcholine esterase level, higher total bile acid levels, higher viral load and longer median hospital stay than those without severe jaundice.[15]
Pregnant patients had higher leukocyte count on admission and also liver enzymes were elevated as compared to non-pregnant group. Jaundice <5 mg/dl was seen in only 3% of pregnant women. However in non-pregnant group, 25% patients had bilirubin level upto 5 mg/dl. It was further seen that 38.5% of pregnant patients had serum bilirubin levels between 11 and 15 mg/dl and 36.5% had bilirubin between 6 and 10 mg/dl. Mean serum bilirubin level was 11.9 mg/dl in pregnancy but 9.2 mg/dl in non-pregnant patients. Thus non-pregnant patients tend to develop milder jaundice i.e., bilirubin in the range of 1-10 mg/dl. While pregnant patients tend to develop deeper jaundice between 06 and 15 mg/dl of range and this difference is statistically significant. HEV infected patients with severe jaundice had significantly lower peak serum levels of γ-glutamyl-transpeptidase, lower albumin levels, lower acetylcholine esterase level, higher total bile acid levels, higher viral load and longer median hospital stay than those without severe jaundice.[15]
Medical complications
Encephalopathy was found to be the most common complication of acute HEV infection in pregnancy. Coagulopathy was the most common complication in non-pregnant group. Other complications in were ascites, GI bleed, renal failure, and seizures. There is no significant difference in occurrence of particular complications between the two groups. A previous analysis[7] showed that, at the time of admission to the intensive care unit, 3 (7.1%), 17 (40.5%), 16 (38.1%) and 6 (14.3%) patients had with grades I, II, III and IV hepatic encephalopathy, respectively. Out of 42 patients clinical coagulopathy was observed in 23 patients, four of whom had disseminated intravascular coagulation (DIC). In four of patients with acute liver failure and intra-uterine fetal death, DIC was noted as early as 12 h after fetal death. Ascites (02), GI bleed (06), hypoglycemia (14), renal failure (01), seizures (04) were other complications noted.
Maternal outcome
We noted that, out of 52 pregnant cases 35 (67%) survived and 17 (32%) cases died. In non-pregnant group 40 (90%) patients survived and only 4 (9%) patients died. This difference was statistically significant. It has previously been observed that maternal mortality was higher in HEV-infected women and occurred exclusively in women with fulminant hepatic failure.[14] Fulminant hepatic failure was more common among HEV-infected women than non-HEV-infected women. Hepatitis is one of the most important medical conditions, due to which patients need obstetric critical care.[17] Out of 42 pregnant women studied by Banait et al.,[13] 23 died (54%), 18 had spontaneous normal delivery and one patient had pre-term delivery with neonatal death. In endemic countries, acute hepatitis E in pregnant women can lead to spectrum of hepatic dysfunction from acute liver failure to decompensation of liver cirrhosis and it is associated with 80% mortality despite the best possible care.[18] In this clinical context, acute viral hepatitis E is the leading cause of a wide spectrum of liver disease ranging from severe acute viral hepatitis, fulminant hepatic failure, to decompensation of liver in cirrhotics
Fetal outcome
In our study, around 28.8% mothers had live birth; 23.1% had pre-term delivery and 48.1% had poor fetal outcome. Only 3% patients had the abortion and could not continue the pregnancy beyond 28 weeks. Analysis of those pregnant women who died due to acute HEV infection, in a study by Beniwal et al.,[12] 16 of 18 (88.9%) cases of HEV died undelivered whereas 5/6 (83.3%) cases in non-HEV group died undelivered. HEV adversely affects both maternal and fetal outcome, with high mortality rate, increased frequency of abortions, pre-term delivery, stillbirth and neonatal death. Pre-term gestation has been noted to occur in pregnant women with hepatitis E.[19] There were 69% fetal deaths and 54% maternal deaths in a study by Banait et al.[13]
We conclude that there is significantly higher occurrence of hepatitis E infection in pregnant women than in non-pregnant women. Incidence of infection goes on increasing as duration of gestation increases. During pregnancy there is a high probability of development of fulminant hepatic failure than in the non-pregnant state. Mortality is higher in pregnant patients compared to non-pregnant patients. About half of pregnant women with hepatitis E have poor fetal outcome in the form of intra-uterine fetal death and stillbirth.
Implications of the study
This is the first study from central India comparing clinical and laboratory profile between pregnant and non-pregnant women with acute HEV infection and describing maternal and fetal outcomes in pregnant women who develop hepatitis E. As we observed that the incidence of infection increases with duration of gestation, it is suggested that pregnant women should be periodically screened for clinical features of acute hepatitis during antenatal visits and should be investigated for hepatitis E in endemic areas. As the disease outcome is poor in pregnant women, an early diagnosis and prompt management is the key. Pregnant women with hepatitis E should be closely monitored for fetal well-being and signs of fetal distress, as this disease also adversely affect the fetal outcome.
Limitations of our study include that this was a hospital based study with a limited sample size. A large prospective study on this topic would throw further light on the disease pattern and outcome of acute hepatitis E in pregnant and non-pregnant women.
Source of Support
Nil.
Conflict of Interest
None declared.
References
- Teshale EH, Hu DJ, Holmberg SD. The two faces of hepatitis E virus. Clin Infect Dis 2010;51:328-34.
- Navaneethan U. Seroprevalence of hepatitis E infection in pregnancy-More questions than answers. Indian J Med Res 2009;130:677-9.
- Gurley ES, Halder AK, Streatfield PK, Sazzad HM, Huda TM, Hossain MJ, et al. Estimating the burden of maternal and neonatal deaths associated with jaundice in Bangladesh: Possible role of hepatitis E infection. Am J Public Health 2012;102:2248-54.
- Khanna A, Hemming AW. Fulminant hepatic failure: When to transplant. Surg Clin North Am 2010;90:877-89.
- Naik SR, Aggarwal R, Salunke PN, Mehrotra NN. A large waterborne viral hepatitis E epidemic in Kanpur, India. Bull World Health Organ 1992;70:597-604.
- Navaneethan U, Al Mohajer M, Shata MT. Hepatitis E and pregnancy: Understanding the pathogenesis. Liver Int 2008;28:1190-9.
- Mufti AR, Reau N. Liver disease in pregnancy. Clin Liver Dis 2012;16:247-69.
- Abraham P. Viral hepatitis in India. Clin Lab Med 2012;32:159-74.
- Devhare PB, Chatterjee SN, Arankalle VA, Lole KS. Analysis of antiviral response in human epithelial cells infected with hepatitis E virus. PLoS One 2013;8:e63793.
- Tripathy AS, Das R, Rathod SB, Arankalle VA. Cytokine profiles, CTL response and T cell frequencies in the peripheral blood of acute patients and individuals recovered from hepatitis E infection. PLoS One 2012;7:e31822.
- Srivastava R, Aggarwal R, Jameel S, Puri P, Gupta VK, Ramesh VS, et al. Cellular immune responses in acute hepatitis E virus infection to the viral open reading frame 2 protein. Viral Immunol 2007;20:56-65.
- Beniwal M, Kumar A, Kar P, Jilani N, Sharma JB. Prevalence and severity of acute viral hepatitis and fulminant hepatitis during pregnancy: A prospective study from north India. Indian J Med Microbiol 2003;21:184-5.
- Banait VS, Sandur V, Parikh F, Murugesh M, Ranka P, Ramesh VS, et al. Outcome of acute liver failure due to acute hepatitis E in pregnant women. Indian J Gastroenterol 2007;26:6-10.
- Patra S, Kumar A, Trivedi SS, Puri M, Sarin SK. Maternal and fetal outcomes in pregnant women with acute hepatitis E virus infection. Ann Intern Med 2007;147:28-33.
- Xu B, Yu HB, Hui W, He JL, Wei LL, Wang Z, et al. Clinical features and risk factors of acute hepatitis E with severe jaundice. World J Gastroenterol 2012;18:7279-84.
- Alam S, Azam G, Mustafa G, Azad AK, Haque I, Gani S, et al. Natural course of fulminant hepatic failure: The scenario in Bangladesh and the differences from the west. Saudi J Gastroenterol 2009;15:229-33.
- Bhadade R, De’ Souza R, More A, Harde M. Maternal outcomes in critically ill obstetrics patients: A unique challenge. Indian J Crit Care Med 2012;16:8-16.
- Mamun-Al-Mahtab, Rahman S, Khan M, Karim F. HEV infection as an aetiologic factor for acute hepatitis: Experience from a tertiary hospital in Bangladesh. J Health Popul Nutr 2009;27:14-9.
- Shrestha P, Bhandari D, Sharma D, Bhandari BP. A study of viral hepatitis during pregnancy in Nepal Medical College Teaching Hospital. Nepal Med Coll J 2009;11:192-4.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.