Comparison the Survival Rate of Fludarabine, Chlorambucil and Cyclophosphamide as First-Line Therapy in Patients with Chronic Lymphocytic Leukemia
2 Department of Haematology & Oncology, Tehran University of Medical Sciences, Tehran, Iran
Sarem Fertility and Infertility Research Center, Sarem hospital, Tehran, Iran
Citation: Sedaghat S, et al. Comparison the Survival Rate of Fludarabine, Chlorambucil and Cyclophosphamide as First-Line Therapy in Patients with Chronic Lymphocytic Leukemia. Ann Med Health Sci Res. 2018;8:99-104
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: Chronic lymphocytic leukemia (CLL) is one of the chronic lymphoproliferative disorders. Many drugs were used for treatment of CLL, but efficacy of them is controversy. In present study, we compare survival of patients with CLL in three treatment regimens of fludarabine, chlorambucil and cyclophosphamide. Methods & Materials: In this historical cohort study, 83 patients with CLL whom participated in imposed war, enrolled to study. Of these, 39 patients received fludarabine (F), 23 patients received prednisone plus chlorambucil (C), and 21 patients received CAP (cyclophosphamide, doxorubicin, and prednisone). Overal survival (OS), Progression Free Survival (PFS), Complete Remission (CR), Partial Remission (PR) and Overall Remission (OR) were evaluated. For comparison, data entered to SPSS-18 & has been analyzed. P-value<0.05 were significant. Result: With a median follow up of 51 months, 16 (19.3%) patients died. Of this, 6 received Fludarabine, 5 received Chlorambucil, and 5 received CVP (P=0.688). PR rates in F, C, and CVP were 53.8%, 30.4% and 42.9%, respectively (p=0.039). CR rates in F, C and CVP group were 23.1, 21.7 and 23.8 percent, respectively (p=0.986), and OR rates were 76.9, 52.2 and 66.7, respectively (p=0.132). Conclusion: According to the results of this study, survival and CR were similar between the three arms of the study. But PR was higher in patients received fludarabine. Comparing results of present study to others, fludarabine has more efficacies on CLL and also suggested as first line treatment.
Keywords
Chronic lymphocytic leukemia; Fludarabine; Chlorambucil; Cyclophosphamide
Introduction
Chronic lymphocytic leukemia (CLL) is one of the chronic lympho-proliferative diseases. CLL is the most common type of leukemia in the world, which is known as a disease of the elderly. However, it is not unusual to diagnose the disease in younger individuals. [1] The incidence of disease increases rapidly after 55 years of age. Unlike other types of leukemia, which are fatal if not treated, the CLL is slowly progressing. But, in some cases, treatment appears to be necessary in these patients. Appropriate treatment for patients with CLL has been the focus of attention for researchers and physicians, and the results presented are confirmed in some similar studies, and questioned in other cases. [2] Treatment options include purine analogues (e.g. Fludarabine and Pentostatin), alkylating agents (e.g. Chlorambucil and Bendamudtin), monoclonal antibodies (such as Rituximab and Alemtuzumab) or a combination of these compounds. [3] In prospective randomized controlled trials, most of these regimens have been compared with chlorambucil as an alkylating agent and a standard treatment in the decades before the emergence of new drugs. [4,5] Having similar overall survival in different regimes, the complete remission rate, the duration of progression and the associated toxicity were different. [6] Despite many studies on comparison of these drugs, the results have been different and somehow inconsistent. [7] Accordingly, this study was designed to compare the survival rate in three treatment regimens of fludarabine, chlorambucil and cyclophosphamide in patients with chronic lymphocytic leukemia.
Materials and Methods
In this historical cohort study, all patients with CLL, whose CLL diagnosis was confirmed from 1999 to 2011, were included in the study. All the patients had the history of admission in Hematology and Oncology wards of Babol hospitals, northern Iran. To determine the exact time of diagnosis, their records were reviewed. All of these patients after discharge had referred to private clinics for follow-up. In private clinics, a case file was made for each of them, and on each visit occasion, new experiments, symptoms and complications from the last visit to the current examination, and if necessary, the performed treatments were recorded in the patients’ records. During a 12- year period of studying, 116 patients with CLL were enrolled in the study that based on inclusion and exclusion criteria, 83 patients whom treated with fludarabine, chlorambucil or cyclophosphamide regimen, remained in the study. All the patients had received no treatment before the mentioned treatments. The patients that their treatment failure has led to start a new therapy were excluded from the study.
The remaining 83 patients in the study were divided into three groups. The first group (F) included 39 patients who had received fludarabine. Fludarabine administration included 25 mg/m2 of fludarabine, which was administered intravenously for five consecutive days and repeated every 28 days for 4-6 cycles.
The second group consisted of 23 patients who received chlorambucil (C). The chlorambucil was prescribed as 10 mg/ m2 orally every 28 days for seventh cycles associated with prednisone as 45 mg per square meter for 5 periods.
The third group consisted of 21 patients who were treated with Cyclophosphamide regimen, Vincristine (Oncovin) and prednisone (CVP or COP). These patients received 650 mg/m2 of cyclophosphamide for one day, 1.5 mg/m2 of Vincristine for the next day, and 45 mg/m2 of prednisone for 5 days, every 28 days for 8 cycles.
Response criteria for patients with CLL were determined according to NCI/WG and IWCLL Guidelines. [8,9]
For complete remission, all the following criteria need to be met for at least 3 months after completion of therapy: [8]
• Lack of basic symptoms associated with CLL (such as weight loss more than 10 percent during the past six months, fatigue causing impairment in everyday life, fevers over 38 °C for more than 2 weeks, night sweats for more than a month)
• Absence of any lymph node with a diameter greater than 1.5 cm on physical examination
• Lack of hepatomegaly or splenomegaly in clinical examination
• Neutrophil count greater than 1500 per mL
• Platelet count greater than 100,000 per microliter
• Hemoglobin greater than 11 g per deciliter
• Lack of lymphocyte clones in peripheral blood by immunophenotyping
Partial remission refers to conditions in which at least one of the following criteria exits for at least two months after the end of treatment: [8]
• At least 50% reduction in peripheral lymphocyte count compared to the pre-treatment count
• At least 50% reduction in the number of nodes enlarged previously; without any increase in their size (Increase less than 25% in lymph nodes under 2 cm is not considered significant) and lack of any enlarged lymph nodes
• If liver and spleen had enlarged before the treatment, their size should have decreased at least 50-percent in touching, ultrasound or CT scan.
• In addition to the above parameters, one of the following hematological parameters should be present
• Neutrophil count greater than or equal to 1500 per ml or more than 50 percent remission compared to the original count without giving GCSF
• Platelet count greater than or equal to 100,000 per microliter or at least 50% improvement compared to the original count
• Hemoglobin concentration greater than or equal to 11 grams per deciliter or 50% improvement over baseline without blood transfusions or giving erythropoietin
Progressive disease refers to the presence of at least one of the followings: [8]
• The appearance of the newly enlarged lymph node (more than 1.5 cm), splenomegaly or hepatomegaly
• 50% or greater increase in the size of the affected area (for example, lymph nodes, spleen or liver). The largest axis of the lymph node, which was before the 1-1.5 cm, should have increased as 50% or more to reach to more than 1.5 cm. Also, the lymph node that its major axis was previously 1.5 cm must be greater than 2 cm.
• Over 50% or greater increase in total circulating lymphocytes with at least 5000 lymphocytes per microliter
• Richter Transformation Report (incidence of aggressive large cell lymphoma) in the lymph node biopsy
• The incidence of neutropenia, anemia, and thrombocytopenia associated with CLL. As administration of cytotoxic agents can cause cytopenia, the cytopenia cannot be used to assess the disease progression. Cytopenia occurring at least three months after completion of treatment also accompanied by clonal infiltration of CLL cells in bone marrow biopsy can be considered as the disease progression. Changes very important in determining the disease progression include a decrease more than 2 g per deciliter in hemoglobin levels or reaching to less than 10 g per deciliter, or more than 50% decrease in platelet count or reaching to less than 100,000 per microliter.
The patients lacking criteria for complete remission, partial remission or a progressive disease are classified in the constant stage of the disease. Therapeutically, the stability of disease is considered as non-responsiveness.
Disease relapse occurs in patients that have achieved complete or partial remission previously based on the above criteria, but after six months or more, the progressive disease will occur.
Information such as age at diagnosis, sex, platelet count, lymphocyte count and hemoglobin concentration at the time of diagnosis, prior to treatment, during treatment, one month after treatment, two months after treatment, and three months after treatment were extracted from the patients’ records. Also, the overall survival and the disease progression-free survival were calculated in the studied subjects.
Data were collected and coded. After recording in the designated tables, they were entered into SPSS software, Ver. 18 and were then analyzed. Description of data obtained was performed through the provision of Frequency Tables and the corresponding graphs. Frequency and percentage were used for describing the qualitative characteristics, while the mean and standard deviation were used for quantitative ones. The chi-square test was used to determine the relationship between qualitative variables, while ANOVA and Post Hoc test were applied to compare the means of the three groups; also, the repeated measures test was used to determine the relationship between continuous variables at different stages. Also, the Kaplan-Meier test and log-rank test were used to determine the survival rate and to compare the survival among groups, respectively. The statistical significance level of all tests was considered as 0.05 so that the P-value <0.05, which indicated the statistical significant difference.
Results
Based on the inclusion and exclusion criteria, 83 patients were studied, including 54 males (65.1%) and 29 females (34.9%). At diagnosis, the mean age of patients was 59.53 ± 10.74 years (range 33 to 84 years old). The mean age of patients at diagnosis time in groups F, C and CVP was 55.41 ± 9.32 years, 63.57 ± 10.68 years and 62.76 ± 10.94 years, respectively. The mean age of the three groups showed significant differences (P=0.003). Thus, the average age of the patients in group F was significantly lower than in the two other groups (P=0.008 compared with group C and P=0.024 compared with CVP group). However, the mean age of patients between C and CVP groups showed no significant difference (P=0.963).
With follow-up median of 51 months, 16 patients (19.3%) died. Of these, six patients were in group F, five patients in group C and five patients in the CVP group (P=0.688).
The complete remission rate was 22.9% (19 patients); the partial remission rate was 44.6% (37 patients) and the overall remission rate was as 67.5%. In Table 1, the rates of complete, partial and overall remission of the three treatment groups were compared. The partial remission in F group was significantly higher than in the other groups.
| Variables | CVP | C | F | P-value |
|---|---|---|---|---|
| Complete remission | 23.80% | 21.70% | 23.10% | 0.986 |
| Partial remission | 42.90% | 30.40% | 53.80% | 0.039 |
| Overall remission | 66.70% | 52.20% | 76.90% | 0.132 |
Table 1: Comparison of the rates of complete, partial and overall remission of the three treatment groups.
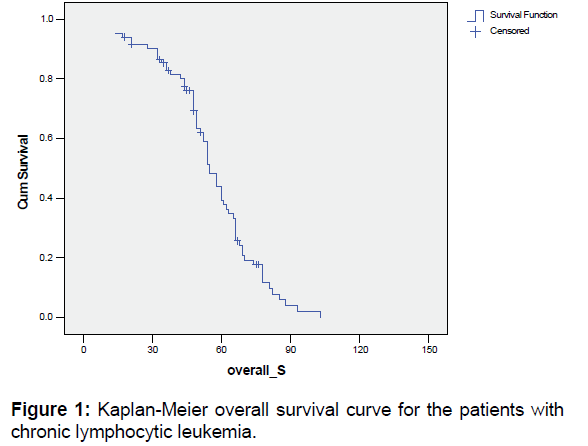
Table 2 and Figure 1 show the overall survival rate and the standard error of studied patients. The median of overall survival of the studied patients was 51 months.
| Months | Kaplan-Meier Estimation | Standard Error |
|---|---|---|
| 12 | 0.95 | 0.02 |
| 24 | 0.90 | 0.03 |
| 36 | 0.82 | 0.04 |
| 48 | 0.69 | 0.05 |
| 60 | 0.39 | 0.06 |
| 72 | 0.19 | 0.05 |
Table 2: The overall survival rate and the standard error of patients with chronic lymphocytic leukemia.
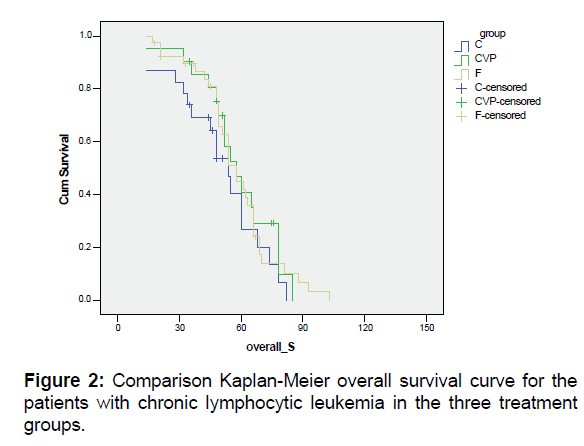
In Table 3, the overall survival rates and SE of the patients with chronic lymphocytic leukemia in the three treatment groups were compared and their Kaplan-Meier curves were specified in Figure 2. The median of overall survival in groups F, C and CVP were 54, 48 and 52 months, respectively. The overall survival in the three treatment groups was not statistically different (P=0.389).
| Months | CVP | C | F |
|---|---|---|---|
| 12 | 0.95 (0.05) | 0.87 (0.07) | 0.97 (0.02) |
| 24 | 0.90 (0.06) | 0.83 (0.08) | 0.92 (0.04) |
| 36 | 0.85 (0.08) | 0.69 (0.09) | 0.87 (0.06) |
| 48 | 0.75 (0.10) | 0.54 (0.11) | 0.74 (0.07) |
| 60 | 0.41 (0.11) | 0.27 (0.11) | 0.42 (0.08) |
| 72 | 0.12 (0.08) | 0.13 (0.08) | 0.14 (0.06) |
Log Rank P-value=0.389
Table 3: The overall survival rates and standard error of the patients with chronic lymphocytic leukemia in the three treatment groups.
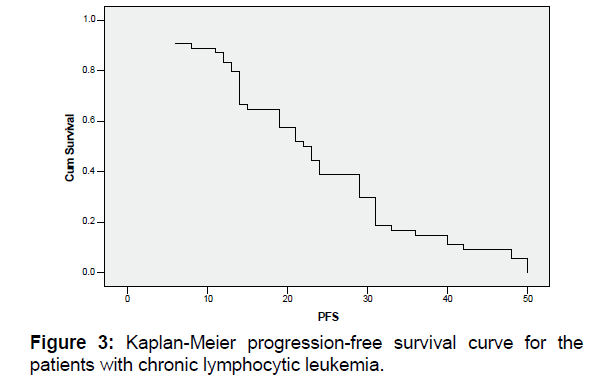
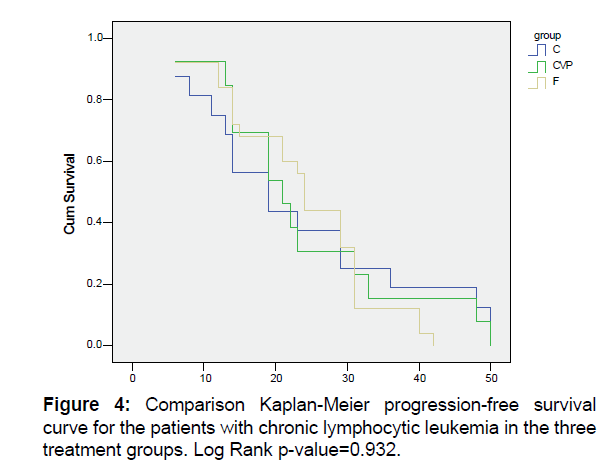
The median of progression-free survival in all studied patients was 22.5 months [Figure 3]. Also, the median of progressionfree survival in groups F, C and CVP were 24, 19 and 21 months, respectively [Figure 4]. The three groups in terms of progression-free survival were not significantly different (P=0.932).
Discussion
In our study, the rates of complete remission, partial remission and overall remission were as 22.9%, 44.6% and 67.5%, respectively. In Asvadi kermani et al. study in Iran, the rates for complete remission and partial remission in patients with CLL reported respectively as 12.7% and 49.2%, while the overall remission rate was 61.9%. [10]
In our study on patients treated with fludarabine, the complete, partial and overall remissions were seen in 23.1%, 58.8% and 76.9% of patients, respectively. In Asvadi kermani study, of all the patients treated with fludarabine, 6% had a complete remission and 50% were partial remission. [10] In Eichhorst study, the complete response rate of 7% and the overall response rate of 83% were reported. [11] The patients treated with fludarabine in Flinn study had complete response of 5% and overall response of 60%. [12]
In our study, the patients treated with chlorambucil showed 21.7% of complete recovery, 30.4% of partial remission and 52.2% of overall remission. In Han study, the rates of complete, partial and overall remission were as 20%, 67% and 87%, respectively. [13] In Hillmen study, the patients received chlorambucil had full recovery of 55% and complete remission of 2%. [14] Hansen et al. reported the 29% of complete remission and 50% of remission in patients treated with Chlorambucil. [15] In Sawitsky study, the overall response rate was equal to 38%. [16] In a study by Robak et al., a complete remission of 12% was reported in patients treated with chlorambucil. [17]
In our study, the rates of complete remission, partial remission and overall remission in the CVP group were as 23.8%, 42.9% and 66.7%, respectively. In Oken study, the overall remission in patients treated with CVP was reported as 52%. [18] Keating reported 25% of complete remission, 40% of partial remission and 65% of overall remission for patients treated with CVP. [19]
In our study, the median overall survival in groups F, C and CVP were respectively as 54 months, 48 months and 52 months. The overall survival in the three treatment groups was not statistically different. Also, the median progression-free survival in groups F, C and CVP were 24 months, 19 months and 21 months. The three groups were not significantly different in terms of progression-free survival. Asvadi kermani in their study calculated the mean survival rate (rather than the median) and reported the mean overall survival of patients under treatment with CVP regimen, chlorambucil and fludarabine as 3.50 months 45 months and 43 months, respectively. [10] In the study by Han, the progression-free survival rate in patients treated with chlorambucil was 12 months. [14] In Keating study, the median survival rate of patients treated with CVP was 5 years. [19] In the French Study Group, the chlorambucil and COP groups had no significant differences in survival rate or disease progression. [20] In a meta-analysis by Landyzynski et al. median overall survival of fludarabine and chlorambucil were 44 and 59 month, respectively. [4] In Eichhorst [11] and Flinn [12] studies, the median progression-free survival rates in patients treated with fludarabine were as 20 months and 19 months, respectively. In a trial study in several countries, fludarabine was compared with CAP regimen. The response rates to fludarabine and CAP in patients not previously treated were as 71% and 60%, respectively. The median duration of remission in patients treated was significantly longer in the fludarabine group. [21] In phase II of the National Cancer Institute study, the effects of fludarabine and chlorambucil were compared in symptomatic patients with CLL. In the fludarabine group, the rates of complete remission, overall remission, progression-free survival and the median of overall survival were respectively as 20%, 63%, 20 months and 66 months, while in the chlorambucil group, the values were as 4%, 37%, 14 months and 56 months. [22] In the phase III of a trial, the effects of fludarabine and chlorambucil were compared in patients with CLL. The complete remission rates in the fludarabine and chlorambucil groups were respectively as 15% and 7%, while the overall remission rate in these two groups was 80% and 72%, respectively. No significant differences were seen between the three groups in rate of overall survival. [23]
Conclusion
Comparing the results of this study with other similar studies, one can conclude that like most of the studies, fludarabine had similar progression-free survival and overall survival rates to chlorambucil and CVP regimens regimen. Also, despite the greater overall remission in patients treated with fludarabine, the difference was not significant. According to the same survival rate and higher rate partial remission in the fludarabine group, it seems that despite some side effects, fludarabine is a better treatment for patients with chronic lymphocytic leukemia than the other two treatment regimens.
Author’s Contribution
All authors have made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data and have been involved in drafting the manuscript or revising it critically for important intellectual content. All authors read and approved the final manuscript.
Financial Support
The present work was supported by a grant from Shiraz University of Medical Sciences. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
Conflict of Interest
All authors disclose that there was no conflict of interest.
REFERENCES
- Emole JN, Locke FL, Pinilla-Ibarz J. An update on current and prospective immunotherapies for chronic lymphocytic leukemia. Immunotherapy. 2015;7:455-466.
- Bazargan A, Tam CS, Keating MJ. Predicting survival in chronic lymphocytic leukemia. Expert Rev Anticancer Ther. 2012;12:393-403.
- Nunes AA, Da Silva AS, Souza KM, Koury CN, De Mello LM. Rituximab, fludarabine, and cyclophosphamide versus fludarabine and cyclophosphamide for treatment of chronic lymphocytic leukemia:A systematic review with meta-analysis. Crit Rev Oncol Hematol. 2015;94:261-269.
- Ladyzynski P, Molik M, Foltynski P. A network meta-analysis of progression free survival and overall survival in first-line treatment of chronic lymphocytic leukemia. Cancer Treat Rev. 2015;41:77-93.
- Cheng MM, Goulart B, Veenstra DL, Blough DK, Devine EB. A network meta-analysis of therapies for previously untreated chronic lymphocytic leukemia. Cancer Treat Rev. 2012;38:1004-1011.
- O'Brien S. New agents in the treatment of CLL. Hematology Am Soc Hematol Educ Program. 2008:457-464.
- Hallek M. Chronic lymphocytic leukemia:2015 Update on diagnosis, risk stratification, and treatment. Am J Hematol. 2015;90:446-460.
- Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia:a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446-5456.
- Eichhorst B, Hallek M, Dreyling M. Chronic lymphocytic leukaemia:ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21:v162-v164.
- Asvadi kermani I, Dehdilani M, Dolatkhah R. Chronic lymphocytic leukemia and effect of fludarabine on treatment of it. J Tabriz Med Uni. 2009;31:17-23.
- Eichhorst BF, Busch R, Hopfinger G, Pasold R, Hensel M, Steinbrecher C, et al. Fludarabine plus cyclophosphamide versus fludarabine alone in first-line therapy of younger patients with chronic lymphocytic leukemia. Blood. 2006;107:885-891.
- Flinn IW, Neuberg DS, Grever MR, Dewald GW, Bennett JM, Paietta EM, et al. Phase III trial of fludarabine plus cyclophosphamide compared with fludarabine for patients with previously untreated chronic lymphocytic leukemia:US Intergroup Trial E2997. J Clin Oncol. 2007;25:793-798.
- Han T, Ezdinli EZ, Shimaoka K, Desai DV. Chlorambucil vs. combined chlorambucil-corticosteroid therapy in chronic lymphocytic leukemia. Cancer. 1973;31:502-508.
- Hillmen P, Skotnicki AB, Robak T, Jaksic B, Dmoszynska A, Wu J, et al. Alemtuzumab compared with chlorambucil as first-line therapy for chronic lymphocytic leukemia. J Clin Oncol. 2007;25:5616-5623.
- Hansen MM, Andersen E, Christensen BE, Christiansen I, Geisler C, Kristensen D, et al. CHOP versus prednisolone + chlorambucil in chronic lymphocytic leukemia (CLL):Preliminary results of a randomized multicenter study. Nouv Rev Fr Hematol. 1988;30:433-436.
- Sawitsky A, Rai KR, Glidewell O, Silver RT. Comparison of daily versus intermittent chlorambucil and prednisone therapy in the treatment of patients with chronic lymphocytic leukemia. Blood. 1977;50:1049-1059.
- Robak T, Blonski JZ, Kasznicki M, Blasinska-Morawiec M, Krykowski E, Dmoszynska A, et al. Cladribine with prednisone versus chlorambucil with prednisone as first-line therapy in chronic lymphocytic leukemia:Report of a prospective, randomized, multicenter trial. Blood. 2000;96:2723-2729.
- Oken MM, Kaplan ME. Combination chemotherapy with cyclophosphamide, vincristine, and prednisone in the treatment of refractory chronic lymphocytic leukemia. Cancer Treat Rep. 1979;63:441-447.
- Keating MJ, Hester JP, McCredie KB, Burgess MA, Murphy WK, Freireich EJ. Long-term results of CAP therapy in chronic lymphocytic leukemia. Leukemia & Lymphoma. 1990;2:391-397.
- French Cooperative Group on Chronic Lymphocytic Leukemia. A randomized clinical trial of chlorambucil versus COP in stage B chronic lymphocytic leukemia. Blood. 1990;75:1422-1425.
- Johnson S, Smith AG, Loffler H, Osby E, Juliusson G, Emmerich B, et al. Multicentre prospective randomised trial of fludarabine versus cyclophosphamide, doxorubicin, and prednisone (CAP) for treatment of advanced-stage chronic lymphocytic leukaemia. The French Cooperative Group on CLL. Lancet. 1996;347:1432-1438.
- Rai KR, Peterson BL, Appelbaum FR, Kolitz J, Elias L, Shepherd L, et al. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. N Engl J Med. 2000;343:1750-1757.
- Catovsky D, Richards S, Matutes E, Oscier D, Dyer MJ, Bezares RF, et al. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the LRF CLL4 Trial):a randomized controlled trial. Lancet. 2007;370:230-239.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.