COVID 19 in Liver Transplant Recipients: Report of 2 Cases
Citation: Omrani S, et al. COVID 19 in Liver Transplant Recipients: Report of 2 Cases. Ann Med Health Sci Res. 2021;11:
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
The COVID-19 epidemic has spread rapidly worldwide. It can cause severe lung disease and multiple organ damage. The support of vulnerable individuals during the CIVID-19 pandemic includes those with congenital or acquired immune-depression. Due to long-term use of oral immunosuppressive drugs and associated comorbidities, it is admitted that liver transplant recipients are suspected to be more susceptible to COVID-19 infection and have a worse prognosis than the general population. In this report, we describe two cases of liver transplant recipient with COVID-19 infection. We also reviewed some literature about the characteristics and management of COVID-19 in liver transplant patients.
Keywords
Liver transplantation; COVID 19
Introduction
The COVID-19 epidemic has spread rapidly worldwide. It can cause severe lung disease and multiple organ damage. The support of vulnerable individuals during the CIVID-19 pandemic includes those with congenital or acquired immunedepression. Due to long-term use of oral immunosuppressive drugs and associated comorbidities, it is admitted that liver transplant recipients are suspected to be more susceptible to COVID-19 infection and have a worse prognosis than the general population.
In this report, we describe two cases of liver transplant recipient with COVID-19 infection. We also reviewed some literature about the characteristics and management of COVID-19 in liver transplant patients.
Case Presentation
Case 1
A 50-year-old man with diabetes mellitus, who underwent orthotropic liver transplantation for Child-Pugh B9 HBV cirrhosis in 2013. The Meld score was 16. He had refractory ascites. The postoperative period was uneventful. He was taking mycophenolate (1000 mg/day) and tacrolimus (4 mg/day). He presented with intermittent fever, weakness and diarrhoea. His vital signs were a temperature at 38°C, a heart rate at 75 beats per minute, a respiration rate at 12 breaths per minute, a blood pressure at 100/60 mmHg. The physical examination was unremarkable. Samples of nasal and pharyngeal swabs for COVID-19 diagnosis were tested using Polymerase Chain Reaction (PCR) which showed positive results. Regular blood tests showed no abnormalities. A CT scan of the chest was not demanded. He did not show any respiratory symptoms. He was put under vitamin C, zinc and paracetamol. His current immunosuppressant dosage was kept. The recovery was confirmed by a negative PCR test realized on the second week post diagnosis.
Case 2
It’s the case of a 45-year-old man who underwent orthotropic liver transplantation in 2009 due to Child-Pugh B9 HBV cirrhosis. The Meld score was 16. He had refractory ascites. His medication included mycophenolate (1000 mg/day), cyclosporine (250 mg/day) and a vitamin K antagonist for deep vein thrombosis. Three months after surgery, he presented with anastomotic biliary stricture. Endoscopic treatment was attempted thrice but with unsuccessful results. Therefore, a biliary digestive anastomosis was performed.
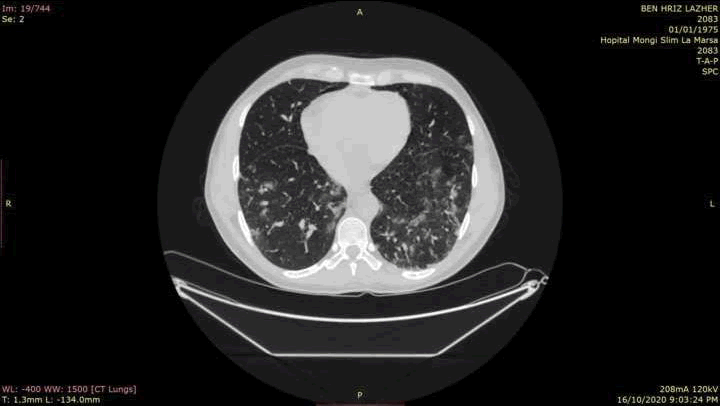
He presented with fever, myalgia, and cough and chest pain. He has no history of diabetes, high blood pressure or coronary artery disease. His vital signs were a temperature at 39°C, a heart rate of 100 beats per minute, a respiration rate of 24 breaths per minute and a blood pressure of 100/60 mmHg. Oxygen saturation on room air was 97%. The rest of the physical examination was unremarkable. Samples of nasal and pharyngeal swabs were taken for COVID-19 evaluation and were tested positive. He was then admitted to the COVID-19 ward. A CT scan of the chest was performed showing multiple peripheral patchy ground glass opacities in both lungs compatible with COVID-19 pneumonia [Figure 1]. He was put under clarithromycin, zinc and paracetamol. In view of the persistence of fever 7 days after admission, we decided to switch antibiotics to levofloxacin. Biological check-up showed severe lymphopenia. Mycophenolate was discontinued. The patient’s fever and cough subsided 2 weeks later. The recovery was confirmed by a negative PCR test performed four weeks after the symptom’s onset. He then resumed mycophenolate after the normalization of lymphocyte count.
Discussion
COVID-19 infection is caused by a new coronavirus named Severe Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) that was first reported in Wuhan, China in December 2019. [1] It was declared as a global pandemic by the World Health Organisation (WHO) on March 11, 2020. It has spread rapidly in China and in other countries worldwide. Many publications have been accumulated trying to improve our understanding of this disease. Yet, very few data are available about the specific risks and characteristics of SARS-CoV-2 infection in liver transplant recipients.
COVID-19 infection usually manifests with symptoms such as fever, dry coughing and sneezing. Transplant recipients are more likely to show atypical clinical presentations such as gastrointestinal symptoms or fatigue. [2] A study showed that upon diagnosis of SARS-CoV-2 infection, 30% of liver transplant recipients had abdominal pain, vomiting or diarrhoea as was the case with our first patient, compared with 12% of patients without liver transplant. [3] Also, liver and kidney injuries were not morefrequent in liver transplant recipients, suggesting that transplanted livers are not more susceptible and that calcineurin inhibitor is not associated with renal injury in SARS- CoV-2 infection. [3] Currently, the impact of immunosuppressant on COVID-19 is still not clear. Some societies have provided recommendations concerning the management of liver transplantation during this epidemic. [4,5] Liver transplant recipients without COVID-19 do not need to adjust the dose of immunosuppressive drugs because of the risk of acute graft rejection. Patients with mild to moderate COVID-19 are to keep current immunosuppressant dosage and the patient’s condition needs to be monitored closely. [4] For patients with severe or rapidly progressing infection, the dosage of calcineurininhibitor has to be reduced. [4] Also, in case of severe lymphopenia, reduction or discontinuation of ant proliferative agents and lymphocyte-depleting therapies like mycophenolate has been suggested. [6] Some studies have reported that hydroxychloroquine has antiviral activity against SARS-CoV-2, so it has been recommended as a potential treatment for COVID-19. [7,8] Some other studies support the use of Azithromycin for its antiviral activity and immunomodulatory effects. [9] However, their use for the treatment of liver transplant recipients with COVID-19 is controversial. [5] Our team decided to initiate hydroxychloroquine in our second patient who presented with severe pneumonia.
Liver transplantation programmes and recipients worldwide have been deeply affected by the coronavirus disease 2019. The transplantation activity has decreased. [6] Many organizations have released recommendations stating that screening is essential and that positive screening results of SARS-CoV-2 testing is seen as a strict contraindication for both organ donation and transplantation to a recipient. [6] They also recommend the reduction of no urgent deceased donor programs especially kidney transplant due to the elective character and existing organ replacement and the reduction of elective living donor programs. [2] This was based on the fact that SARS-CoV-2 mode of transmission is still not fully comprehended. Whether the virus can be transmitted parenterally or via solid non-lung organ transplant is not known. A case of COVID-19- associated hepatitis in the recipient of a living donor liver allograft, whose donor subsequently tested positive for COVID-19, has been described. [10] Furthermore, there’s the potential contamination of the transplant team, the organ procurement organization and associated hospital workforce community, especially in the absenceof effective therapy that pledges in favour of this strategy. At all stages, the transplant procedure requires high capacities of trained staff, operating rooms and intensive care units that, during this pandemic, are needed to manage more urgent cases. Also, post-transplant care includes a high-level medication-inducted immunosuppression to the transplant recipient. Yet, deduced from data on other viruses and SARS, such a state is accompanied with high viral load, prolonged shedding and severe clinical manifestations. [11] The underlying cause might the immunosuppressive drug itself but might as well be the comorbidities that accompany solid organ recipient. [12] In fact, a multicentre cohort study reported results from a direct comparison between contemporaneous cohorts of patients with laboratory-proven SARS-CiV-2 infection with and without liver transplant. Data were collected from two international registries: the COVID-Hep registry coordinated by the University of Oxford (UK) and the SUCURE- Cirrhosis registry coordinated by University of North Carolina (USA). The results showed that liver transplantation was not associated with an increased risk of mortality and that among liver transplant recipients, advanced age, increased baseline creative concentration and the presence of a non-liver cancer were independently associated with an increased risk of mortalities. Yet, the type of immunesuppressants used and the time from transplantation were not independently associated with mortality. [3] Many publications showed that, unlike common viral agents, SARS-CoV-19 might not lead to a worse general condition in immunosuppressed patients. [13] In fact, in some reports, transplanted patients with COVID-19 have fewer complications than expected. [14] One hypothesis is that SARS-CoV-2 may cause disease through the immune system, so the use of immunosuppressive mediations may reduce the complications by inhibiting the immune response. [15]
Conclusion
In conclusion, the COVID-19 pandemic has affected healthcare resources around the world. Some recommendations have been provided but the situation continues to evolve rapidly and these recommendations will need to evolve as well. For this to happen, knowledge and experience should be shared.
REFERENCES
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-733.
- Ritschl PV, Nevermann N, Wiering L, Wu HH, Moroder P, Brandl A, et al. Solid organ transplantation programs facing lack of empiric evidence in the COVID-19 pandemic: A By-proxy Society Recommendation Consensus approach. Am J Transplant. 2020;20:1826-1836.
- Webb GJ, Marjot T, Cook JA, Aloman C, Armstrong MJ, Brenner EJ, et al. Outcomes following SARS-CoV-2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol Hepatol. 2020;5:1008-1016.
- Liu H, He X, Wang Y, Zhou S, Zhang D, Zhu J, et al. Management of COVID-19 in patients after liver transplantation: Beijing working party for liver transplantation. Hepatol Int. 2020;14:432-436.
- Fix OK, Hameed B, Fontana RJ, Kwok RM, McGuire BM, Mulligan DC, et al. Clinical best practice advice for hepatology and liver transplant providers during the COVID-19 pandemic: Aasld expert panel consensus statement. Hepatology. 2020;72:287-304.
- Di Maira T, Berenguer M. COVID-19 and liver transplantation. Nat Rev Gastroenterol Hepatol. 2020;17:526-528.
- Alanagreh L, Alzoughool F, Atoum M. Risk of using hydroxychloroquine as a treatment of COVID-19. JRS. 2020;31:111-116.
- Meo SA, Klonoff DC, Akram J. Efficacy of chloroquine and hydroxychloroquine in the treatment of COVID-19. Research Strategies n.d.:9.
- Bleyzac N, Goutelle S, Bourguignon L, Tod M. Azithromycin for COVID-19: More than just an antimicrobial? Clin Drug Investig. 2020;40:683-686.
- Lagana SM, De Michele S, Lee MJ, Emond JC, Griesemer AD, Tulin-Silver SA, et al. COVID-19 associated hepatitis complicating recent living donor liver transplantation. Arch Pathol Lab Med 2020;144:929-932.
- Kumar D, Manuel O, Natori Y, Egawa H, Grossi P, Han S, et al. COVID-19: A global transplant perspective on successfully navigating a pandemic. Am J Transplant. 2020;20:1773-1779.
- Park JY, Kim MH, Bae EJ, Kim S, Kim DK, Joo KW, et al. Comorbidities can predict mortality of kidney transplant recipients: Comparison with the Charlson comorbidity index. Transplant Proc. 2018;50:1068-1073.
- D’Antiga L. Coronaviruses and immunosuppressed patients: The facts during the third epidemic. Liver Transp. 2020;26:832-834.
- Niknam R, Malek-Hosseini SA, Hashemieh SS, Dehghani M. COVID-19 in liver transplant patients: Report of 2 cases and review of the literature. IMCRJ. 2020;13:317-321.
- Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: Consider cytokine storm syndromes and immunosuppression. The Lancet. 2020;395:1033–1034.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.