Disparities in the Magnitude of Human Immunodeficiency Virus-related Opportunistic Infections Between High and Low/Middle income Countries: Is Highly Active Antiretroviral Therapy Changing the Trend?
- *Corresponding Author:
- Dr. Iroezindu MO
Infectious Diseases Unit, Department of Medicine, College of Medicine, University of Nigeria, Enugu Campus, Enugu State, Nigeria.
E-mail: mikezindu@yahoo.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Citation Iroezindu MO. Disparities in the magnitude of human immunodeficiency virus-related opportunistic infections between high and low/middle-income countries: Is highly active antiretroviral therapy changing the trend?. Ann Med Health Sci Res 2016;6:4-18.
Abstract
Opportunistic infections (OIs) cause significant morbidity/mortality in human immunodeficiency virus (HIV) infected individuals globally. Disparities between high income countries (HICs) and low/middle income countries (LMICs) in the magnitude of HIV related OIs in pre-highly active antiretroviral therapy (HAART) populations was reviewed, and HAART induced decline in OIs was further compared between the two settings. Studies published in English from onset of HIV epidemic up to December 2013 were searched in PubMed, Google, Google Scholar, and African Journal online. An article was included if (a) the study was conducted in HIC or LMIC, (b) the age of the participants was ≥12 years, (c) the HAART status of the participants was stated, and (d) various types of OIs were investigated. In predominantly pre HAART populations, the incidence and prevalence of overall HIV related OIs in HIC ranged from 5.5 to 50.0 per 100 person years (PY) and 27.4–56.7%, respectively. In LMIC, the respective overall incidence and prevalence of OIs were 12.2–93.9 per 100 PY and 32.0–77.7%. Pneumocystis jirovecii pneumonia, candidiasis, Cytomegalovirus disease, Mycobacterium avium complex disease, and Kaposi’s sarcoma were the most frequent OIs in HICs while tuberculosis, candidiasis, chronic diarrhea, and cryptococcosis were predominant in LMICs. The introduction of HAART led to substantial reduction in the incidence of OIs with more impressive percentage decline in HICs (43–97%) compared to 30–79% in LMICs. Disparities in the magnitude of HIV related OIs between HICs and LMICs are evident both in the pre HAART and post HAART era. Efforts to optimize HAART induced decline in HIV related OIs should become a global health priority irrespective of prevailing socioeconomic circumstances.
Keywords
Acquired immune deficiency syndrome, Disparities, High-income country, Highly active antiretroviral therapy, Human immunodeficiency virus, Low-income country, Middle-income country, Opportunistic infections
Introduction
The human immunodeficiency virus (HIV) infection and its progression to the acquired immune deficiency syndrome (AIDS) have significantly affected the global health statistics over the past three decades. Globally, there are an estimated 35.3 million people living with HIV (PLHIV), and 2.3 million new HIV infections and 1.6 million AIDS deaths occur annually.[1] By far, the greatest impact of the disease has been in low- and middle-income countries (LMICs) where an estimated 90% of the global HIV-infected population live.[1]
The hallmark of HIV infection is immunosuppression which predisposes affected individuals to infections by unusual pathogens referred to as opportunistic infections (OIs). OIs constitute a major cause of morbidity and mortality in PLHIV, globally.[2-6] Over the three decades of the HIV epidemic, HIV-related OIs commonly reported in various populations include Pneumocystis jirovecii pneumonia (PJP) (previously called Pneumocystis carinii pneumonia), tuberculosis (TB), candidiasis, Cytomegalovirus (CMV) disease, Mycobacterium avium complex (MAC) disease, chronic parasitic diarrhea, toxoplasmosis, and cryptococcosis.[2,3,7-14] Findings of some studies suggest differences in the magnitude and types of HIV-related OIs seen in LMICs,[7-11] and high-income countries (HICs).[12-14]
Before the introduction of antiretroviral therapy (ART), progression to AIDS and premature death was the reality for HIV-infected individuals in both HICs and LMICs. During that period, AIDS-related deaths where overwhelmingly attributed to OIs. In the era of highly active ART (HAART), a significant decline in the magnitude of OIs as well as overall morbidity and mortality in HIV-infected populations have been demonstrated in various populations.[15-18] Nevertheless, available reports suggest that OIs remain an important cause of morbidity and mortality in PLHIV in the era of HAART in several populations.[19-21] Considering that access to HAART and optimization of antiretroviral treatment options have not been uniform among HIV-infected populations across various socioeconomic divides,[19] the positive impact of HAART in reducing the magnitude of HIV-related OIs may therefore have regional dimensions.
This review has three major objectives: First, to compare the overall incidence (or prevalence) of HIV-related OIs between HICs and LMICs in predominantly pre-HAART populations. Second, to highlight any disparities between LMICs and HICs in the frequency of individual OIs documented in available studies. Finally, to compare the impact of HAART on the magnitude of HIV-related OIs between the two socioeconomic settings.
Materials and Methods
Search strategy
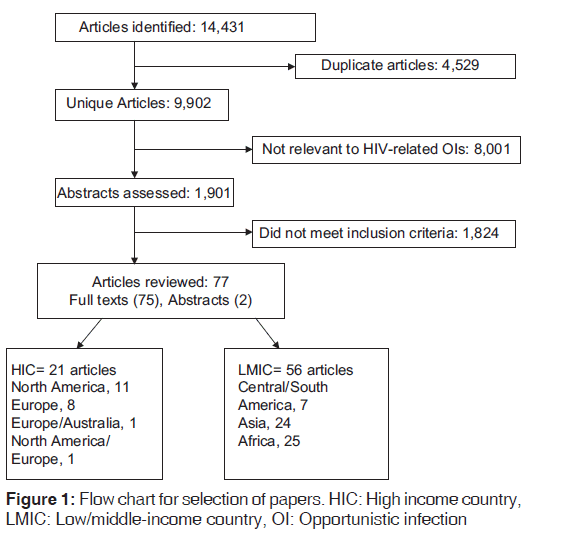
Online databases and search engines including PubMed, Google, Google Scholar, and African Journal on-line were searched for articles published on HIV-related OIs from the onset of the epidemic up to December 31, 2013. Key words and expressions used for the search included “HIV-related opportunistic infections,” “HIV-related opportunistic diseases,” “HIV-associated opportunistic infections,” “HIV-associated opportunistic diseases,” “AIDS-related opportunistic infections,” “AIDS-related opportunistic diseases,” “AIDS-associated opportunistic infections,” “AIDS-associated opportunistic diseases,” and “AIDS-defining illness”. The search was limited to articles published in English. A total of 14,431 articles were initially identified. After elimination of duplicates and studies that were not relevant to OIs in the setting of HIV/AIDS, 2,183 studies remained. Available full articles or abstracts of these studies were scrutinized to identify those that met the inclusion criteria. References of relevant articles were also checked. After exclusion of articles that did not meet the inclusion criteria, 77 articles that met the inclusion criteria were reviewed. The selection flowchart for papers reviewed is shown in Figure 1.
Selection criteria
Studies that met the following criteria were included: (a) Study was conducted in a setting (s) easily identifiable as HIC, LMIC as defined by the World Bank in 2013,[22] (b) age of the participants was =12 years, (c) the HAART status of the study population was stated, (d) the study investigated various types of OIs in an HIV-infected population rather than a single OI.
Ethical considerations
The author did not receive any funding for this review, and there are no personal, political, or academic competing interests. Accurate data extraction was ensured as much as possible. Extracted duplicate publications were deleted, and any other discrepancies were resolved before articles were included in the review. Articles that met the eligibility criteria were included in the review irrespective of whether their findings were positive or negative.
Terminologies
ART-naive population referred to those who did not receive any form of ART.
HAART population referred to individuals who received ART (of any duration) made up of three drugs from at least two antiretroviral classes irrespective of whether a protease inhibitor (PI) was used.
Bearing in mind that classification of HAART status as previously described by Detels et al.,[23] using the 1996 cut-off calendar period (before 1996- pre-HAART; 1996 and beyond- post-HAART) may not be generalizable to several settings in LMICs that were yet to have established access to HAART around 1996, when the terms pre-HAART and post-HAART were used for specific studies, the calender periods being referred to were highlighted.
Chronic diarrhea as used in this review referred to cases mainly caused by cryptosporidium and/or Isospora belli.
HIV-related (or HIV-associated) OI included both AIDS-defining and non-AIDS defining OIs. This general term was adopted to harmonize the differences among the studies. Some studies investigated for only AIDS-defining OIs while others included non-AIDS defining OIs. Among the studies that investigated for only AIDS-defining OIs, various criteria were used such as CDC, 1987;[24] WHO, 1990;[25] CDC, 1993;[26] and WHO, 2005.[27]
Results
Seventy-seven publications met the study’s inclusion criteria. Twenty one studies were conducted in HICs while the remaining 56 were carried out in LMICs. In HICs, there were 14 prospective cohort studies, 5 retrospective cohort studies, 1 randomized control trial and 1 bidirectional study with prospective, and retrospective arms. In LMICs, 20 studies had a prospective cohort design, 14 were retrospective analysis, 20 were cross-sectional studies, 1 was bidirectional (both prospective and retrospective arms), and 1 had both retrospective and cross-sectional designs. The findings of these studies are highlighted below and summarized in Tables 1-3.
Overall magnitude of opportunistic infections
Tables 1 and 2 summarize the studies that reported the overall incidence or prevalence (respectively) of OIs among predominantly pre-HAART populations in both HIC and LMIC. The overall incidence of HIV-related OIs in various studies in the HIC of the United States of America, Western Europe, and Australia ranged from 4.5 to 50.0 per 100 person-years (PY) of follow-up.[15-17,20,28-35] Fewer studies reported the overall prevalence of HIV-related OIs in HICs and found rates of 27 [1,493/5,451]–57 [228/402] %.[12,13,41] The majority of studies in HICs reported only the first episodes of HIV-related OIs[12,15-17,30-32,34,35] while others included any incident OI that occurred during the study.[13,28,33]
In LMICs of Asia, South/Central America, and sub-Saharan Africa, the overall incidence of HIV-related OIs was 12.2–93.9 per 100 PY.[18,36-40] Several studies reported the overall prevalence of OIs in LMICs and the range was 32.0 [66/207]–77.7 [136/175]%.[7-11,42-49] As was the case in HICs, most of these studies reported only the first HIV-related OI events[7,9,10,18,36,38,39,41,44] while others included any incident OI that occurred during the study.[8,40,42,47]
Magnitude of individual opportunistic infections
The frequency or incidence of individual OIs reported in various HICs and LMICs are presented below on the regional basis. In each region, the frequency of individual OIs as a proportion of total OIs documented is presented first followed by reports of incidence rates for individual OIs where available.
North America
In a survey of 6682 HIV-infected patients spanning 1990–1994 in the United States, 1883 died from OIs. The OIs that were most frequently experienced were PJP 45%, MAC disease 25%, CMV disease 23%, and esophageal/pulmonary candidiasis 22%.[2] The predominance of candidiasis (0.5–13.0 per 100 PY), PJP (0.4–9.0 per 100 PY), MAC (0.3–7.0 per 100 PY), CMV (0.2–7.0 per 100 PY), and Kaposi’s sarcoma (KS) (2.5 per 100 PY) among OIs that occurred in predominantly pre-HAART US cohorts was also highlighted by other studies that documented their incidence rates.[15,28,29,51]
Western Europe and Australia
The frequency of individual OIs reported in various studies in Europe was fairly reflective of the predominant pattern in HIC. The frequently reported OIs were: CMV (9–37%),[34,52,53] PJP (10–29%),[34,52,53] toxoplasmosis (2.1–37%),[34,52,53] candidiasis (8–23%),[34,52,53] KS (7–28%),[34,52,53] and MAC (8–17%).[34,52,53] TB (7–11%),[52,53] and cryptococcosis (7%)[52] were less frequently experienced.
The incidence of individual OIs in studies conducted in Europe and Australia were as follows: Toxoplasmosis (1.5–12.6 per 100 PY),[16,33,54] PJP (1.5–11.4 per 100 PY),[16,17,33,35,54] candidiasis (1.7–11.0 per 100 PY),[16,17,33,35,54] MAC (0.37–9.5 per 100 PY),[16,17,33,35,54] CMV (0.59–6.8 per 100 PY),[16,17,33,35,54] TB (0.64–3.6 per 100 PY),[16,17,33,54] and chronic diarrhea (0.6 per 100 PY).[33]
Central and South America
In Central and South America, the frequently reported OIs included KS (5–47%),[46,55] candidiasis (12–44%),[11,46,56,57] TB (9–32%),[11,46,55-57] chronic diarrhea (3–33%),[11,46,55-57] PJP (13–29%),[11,46,55-57] toxoplasmosis (4–18%),[11,55-57] and cryptococcosis (5–14%).[11,55-57] CMV (2–5%),[11,46,55-57] and MAC (1%)[11,56,57] were infrequent conditions. None of the available studies documented incidence rates for individual OIs.
Asia
The OIs that were more frequently documented in Asia included candidiasis (1–88%),[3,7,10,18,43-45,58-64,67] TB (11.3–71%),[3,7,8,10,42,44,45,58,59,62-66,68-71] chronic diarrhea (0.3–47%),[3,7,8,10,43-45,58-67,69] CMV (1–45%),[3,7,10,18,43-45,58-64,67,71] and cryptococcosis (2–38%).[3,7,8,10,18,42,44,58,59,62-64,66,67] Other conditions were less frequently reported including PJP (1–23%),[3,8,10,42,58,59,62,63,67,68] toxoplasmosis (1–11%),[3,8,10,18,58,64,66,67] and MAC (2–3%).[64,65] KS was not documented in the available studies.
The incidence of individual HIV-related OIs was documented by Ghate et al.[36] in 457 cohorts in India. TB was the most common condition with an incidence of 15.4 per 100 PY, followed by candidiasis (11.3 per 100 PY). Cryptococcal meningitis was relatively less common at 1.7 per 100 PY while the incidence of each of PJP, CMV, and toxoplasmosis were < 1.7 per 100 PY.
Sub-Saharan Africa
In sub-Saharan Africa, the frequency of individual OIs were as follows: TB (13–64%),[6,9,22,47-49,72-84] candidiasis (11–42%),[9,48,49,75-79,81,83] cryptococcosis (1–39%),[9,22,47,49,74-79,81,83-85] chronic diarrhea (3–35%),[9,22,47,49,75-81] toxoplasmosis (2–24%),[9,47,78-81] KS (1–18%),[9,22,49,72,77,78,82] CMV (1–18%),[9,75,77,82] and PJP (0–5%).[22,47,76-81] MAC was not documented in any of the available studies in sub-Saharan Africa. None of the available studies documented incidence rates for individual OIs.
Impact of highly active antiretroviral therapy on the magnitude of human immunodeficiency virus-related opportunistic infections
Impact on incidence or prevalence of overall opportunistic infections
Since the introduction of HAART, a significant decline in AIDS progression and the burden of OIs have been observed globally.[8,9,16,83-86] Table 3 summarizes the findings of cohort studies that documented the effect of HAART on the overall incidence or prevalence of HIV-related OIs.
In the HICs of North America, Western Europe and Australia, the introduction of HAART led to the significant decline of about 42.8–96.6% in the overall incidence of HIV-related OIs.[14-17,20,30-35] In the LMIC of South/Central America, Asia and sub-Saharan Africa, the decline in the overall incidence of OIs was about 30.0–79.0%.[11,18,36,37,39] In three studies that reported the effect of HAART on the overall prevalence of OIs in LMIC, the percentage reduction was about 38.3–47.0%.[8,9,46,50] As shown in Table 3, the HAART-induced decline in the overall incidence of OIs in Ivorian cohorts was seen for the first, second and third events with the percentage decline in OI events higher for subsequent events.[39] De Beaudrap et al.[9] in Senegal found that the overall incidence of OIs dropped from 20.5 per 100 PY in the 1st year of HAART to 4.3 per 100 PY in the 4th year post-HAART. However, unlike what was seen in several other populations that observed a progressive decline in OIs when patients on HAART were followed up on a long-term basis, the incidence of OIs in the Senegalese cohorts began to increase by 5% per month after the 4th year of HAART.
Magnitude of individual opportunistic infections in the post-highly active antiretroviral therapy era
Generally, the magnitude (incidence or prevalence) of individual OIs among HIV cohorts post-HAART was reported by few studies. In HICs, the cohort studies that reported incidence of individual OIs post-HAART found the following rates per 100 PY: Candidiasis 1.0–5.7,[16,17,33,35] MAC 0.6–2.7,[16,17,33,35] CMV 0.6–5.1,[16,17,33,35] PJP 0.2–4.4,[16,17,33,35] KS 0–2.3,[16,17,33,35] TB 0.2–2.6,[16,17,33,35] toxoplasmosis 0.2–1.8,[16,33,35] and cryptococcosis 0.6.[33] The frequency of individual OIs in HICs reported in two cohort studies were: CMV 1.8–7.7%,[15,34] PJP 3.9–11%,[15,34] MAC 2.5–6.2%,[15,34] candidiasis 5.4–19%,[15,34] KS 1.2–8%,[15,34] TB 0.8–1.9%,[15,34] toxoplasmosis 0.5–1.3%,[15] and chronic diarrhea 0.8–2%.[15]
In four cohort studies, the following frequency of individual OIs among HIV-infected patients post-HAART in LMICs was found: TB 13.0–38.2%,[8,9,11,18] candidiasis 11.0–23.1%,[8,9,18] cryptoccosis 0–20.6%,[8,9,11,18] CMV 0–23.1%,[8,9,11,18] PJP 0–29.5%,[8,9,11,18] KS 0–5.2%,[8,9] MAC 15.4–18.2%,[8] and toxoplasmosis 0–8.8%.[8,9,11,18]
Retention in human immunodeficiency virus care
Retention in HIV care is required for optimal clinical outcomes including reducing the magnitude of OIs. Available literature shows that definitions of retention in HIV care are rather heterogenous. According to the WHO, “Retention in HIV care” can be defined from the moment of initial engagement in care, when a person with HIV is linked successfully to services, to assessment for eligibility, initiation on ART, and retention in lifelong ART care.[87] However, in other studies and reports, it sometimes includes the period from diagnosis to successful linkage to care.[87]
Some studies in Western countries have assessed retention in care from such comprehensive perspectives. In a meta-analysis of 28 US studies with a total of 75, 655 HIV-diagnosed persons, it was reported that 59% of them, regardless of the length of time since diagnosis, were retained in care.[88] Retention in care was defined regarding multiple HIV medical care visits averaged across the assessment intervals. The estimate increased to 62% after omitting the two studies that had long assessment intervals of 3 and 5 years. The percentage of HIV-diagnosed persons retained in care was higher in studies conducted before 2003 (62%) than in studies conducted in 2003 or more recently (42%).[88]
In the United Kingdom, the 12-month retention rate of all 72,840 adults seen for HIV care in 2011 was 95%.[89]
In HIV treatment programs in resource-limited settings, retention in care has been defined as “patients known to be alive and on ART at the end of a follow-up period either at the same facility or formally transferred out to another ART unit and thus assumed to be on therapy.”[90,91] A large systematic review in 2007 that surveyed 32 publications on 33 cohorts comprising 74,289 patients in 13 countries reported a plausible mid-point estimate of retention 2 years after ART initiation of 50% among HIV-infected patients on ART in Africa.[90] The analysis was updated in 2010 using an additional 39 cohorts of 226, 307, and it was found that the 24-month retention rate was 70.0%.[92]
Considering that most reports of retention in HIV care are based of clinic perspectives, it is likely that patients who are lost to follow-up have been assumed to have disengaged from care. However, in the setting of rapid ART scale-up and decentralization of care, this assumption may not always be true.[93] Some studies in Africa have shown that 50–55% of HIV-infected patients on ART who were lost to follow-up and found to be alive following contact tracing were still in care elsewhere.[94,95]
So far, there is a paucity of literature on long-term retention on ART (after 24 months), making this an important area for further study, especially in the light of recent changes in the WHO treatment guidelines, recommending treatment initiation at =500 cells/µl.
Discussion
In this review, the magnitude of HIV-related OIs in predominantly pre-HAART populations in both HIC and LMIC were described, and the percentage decline in the magnitude of OIs following the introduction of HAART was further highlighted in the two socioeconomic settings. The incidence or prevalence of overall HIV-related OIs was generally high in predominantly pre-HAART populations in both settings, but LMIC appeared to have higher OI incidence and prevalence. There were also disparities in the types of OIs predominant in each socioeconomic setting. While PJP, candidiasis, CMV disease, MAC disease, KS, and occasionally toxoplasmosis were predominant in HICs; TB, candidiasis, chronic diarrhea, and cryptococcosis were consistently more frequent in LMICs. Substantial reductions in the overall incidence or prevalence of OIs were documented following the introduction of HAART both in HICs and LMICs, but the percentage decline appeared more impressive in HICs.
There are a number of reasons for the apparent disparities in HIV-related OIs between HICs and LMICs. First, the methodological differences in the various studies reviewed could account for this. While the majority of the findings of HICs were based on the large prospective cohort studies, studies in LMICs mainly involved prospective or retrospective cohorts, or some cross-sectional studies with relatively smaller sample sizes. There were also some subtle differences in the definition of diseases selected for inclusion as OI in the various studies. In some studies, there were immunological criteria for inclusion, and this would strongly influence the spectrum of OIs since certain OIs are more commonly encountered with more severe degrees of immunosuppression. There is overwhelming evidence that low baseline CD4+ cell count[16,36,54,64,96-99] and low CD4+ cell count during ART[16,29,100] are strong risk factors for OIs both in HICs and LMICs.
Beyond issues that bother on methodology, it has been shown that geographical differences may have a role to play in the reported incidence of HIV-related OIs.[54] This may explain why PJP and KS appeared relatively more frequent in Central/ South America than the other LMICs of Asia and sub-Saharan Africa. Nevertheless, the apparent rarity of MAC, CMV, and sometimes PJP in LMICs possibly raises concern on the role of local diagnostic capacity in the magnitude of reported OIs. The fact that a good number of centers in LMICs, especially sub-Saharan Africa do not have sufficient diagnostic facilities for these three conditions should be borne in mind before drawing strong inferences on the burden of these OIs in LMICs.
The role of racial factors in predicting the incidence or types of OIs in HIV-infected populations have also been investigated. In an evaluation of the profile of OIs in HIV-infected persons living in Australia,[101] PJP was clearly, the most commonly diagnosed condition in people of all country/region of birth except for individuals born in sub-Saharan Africa. Those born in sub-Saharan Africa had an increased risk of TB and cryptococcosis but a decreased risk of PJP and esophageal candidiasis compared to individuals born in Australia even after adjusting for length of stay in Australia. TB risk was also higher among those born in Asia-Pacific and other low-income countries, but the risk of various types of OIs was similar for HIV-infected patients born in Australia and other industrialized countries. According to Del Amo et al.,[102] TB accounted for 27% of the initial episodes of AIDS-related OIs in Africans but 5% in non-Africans while PJP was the initial AIDS-related condition in 34% of non-Africans but 17% for Africans. In Netherlands, it was reported that compared to patients from Western Europe, Australia, and New Zealand, patients of sub-Saharan African origin had a significantly lower risk for PJP both at the time of HIV diagnosis and during follow-up while in care after adjusting for confounders.[98] The authors suggested that differences in genetic susceptibility may partially explain the lower PJP incidence in the African patients.
The role of racial factors in predicting the incidence or types of OIs in HIV-infected populations have also been investigated. In an evaluation of the profile of OIs in HIV-infected persons living in Australia,[101] PJP was clearly, the most commonly diagnosed condition in people of all country/region of birth except for individuals born in sub-Saharan Africa. Those born in sub-Saharan Africa had an increased risk of TB and cryptococcosis but a decreased risk of PJP and esophageal candidiasis compared to individuals born in Australia even after adjusting for length of stay in Australia. TB risk was also higher among those born in Asia-Pacific and other low-income countries, but the risk of various types of OIs was similar for HIV-infected patients born in Australia and other industrialized countries. According to Del Amo et al.,[102] TB accounted for 27% of the initial episodes of AIDS-related OIs in Africans but 5% in non-Africans while PJP was the initial AIDS-related condition in 34% of non-Africans but 17% for Africans. In Netherlands, it was reported that compared to patients from Western Europe, Australia, and New Zealand, patients of sub-Saharan African origin had a significantly lower risk for PJP both at the time of HIV diagnosis and during follow-up while in care after adjusting for confounders.[98] The authors suggested that differences in genetic susceptibility may partially explain the lower PJP incidence in the African patients.
HIV-infected patients on HAART who have poorer socioeconomic living conditions have been found to have higher risk of OIs. Iroezindu et al.[96] found that poor household income was an independent predictor of OIs among Nigerian patients on HAART. Badri et al.[103] also reported that low socioeconomic status was significantly associated with incident TB during HAART in South Africa. While poor living conditions may not be a critical problem in HICs, failure to determine the effect of poverty on the development of OIs in the era of HAART in LMICs would be a fundamental oversight.
The findings of this review have important regional and global public health implications in the management of HIV/AIDS. First, the efficacy of HAART in reducing OI morbidity and its attendant mortality in PLHIV has been shown beyond reasonable doubt irrespective of socioeconomic differences. However, despite emerging evidence of the significant burden of noninfective morbidity in PLHIV in a number of studies.[104,105] OIs continue to be a problem even in the era of HAART as the burden of disease is still considerable in several populations. Since the somewhat more impressive percentage decline in OI burden reported in HICs compared to LMICs may not be unconnected with the earlier, wider, and more durable access to HAART, it re-emphasizes the need for intensification of the scaling up of ART in resource-limited settings.
The findings of this study should be interpreted in the light of its strengths and limitations. One of the strengths of this review is that the period considered spanned the entire three decades of the HIV/AIDS epidemic. Moreover, the studies included in this review involved patients in all the continents of the world. In addition, the majority of the studies reviewed reported incidence rates which adjusted for population size and period under exposure thereby allowing for some reasonable comparisons between studies with different sample sizes. A major limitation of this review regarding the overall incidence or prevalence of OIs is the differences in the definition of diseases selected for inclusion as OI in the various studies. While some studies investigated only AIDS-defining conditions, others included non-AIDS-defining OIs depending on the peculiarities of their geographical region. Second, there were relatively fewer studies from LMICs (especially sub-Saharan Africa) that performed active follow-up over long periods of time compared to HICs. In addition, in some countries or regions, only one or two studies published in English were found, and these studies may not necessarily be representative of the picture in the entire country or region. As a result of the unavailability of information regarding chemoprophylaxis for OIs in the majority of studies, this review was unable to assess the possible impact of OI chemoprophylaxis on the changes in OI incidence following the introduction of HAART. The lack of diagnostic capacity for certain OIs such as MAC and CMV diseases in several resource-limited settings also calls for caution while drawing conclusions on the actual incidence or prevalence of these OIs in LMICs.
Conclusion
This review found that the magnitude of HIV-related OIs was substantial in both HICs and LMICs before the introduction of HAART, but LMICs appeared to have higher OI incidence or prevalence. There were also disparities in the types of OIs frequently reported in each socioeconomic setting. It was further shown that the introduction of HAART led to substantial reductions in the overall incidence of OIs with somewhat more impressive impact in HICs. As our clinical concerns increasingly shift towards the growing burden of noninfective complications of HIV and its treatment, let us not erronously conclude that OIs have become a thing of the past. According to Brooks et al.,[106] “OIs are here to stay, and they will continue to demand our attention for the foreseeable future. They continue to occur among patients who are unaware of their HIV infection and among patients who, despite awareness of their HIV infection, remain unlinked to care, or if receiving care, are unable to assess optimal ART for several reasons.” Incidentally, nearly all the factors that perpetuate OIs in the era of HAART as observed by Brooks et al.[106] are much more prevalent in LMICs. Should it then be appropriate to say that the achievements of the scaling up of ART in resource-limited settings may face a major threat in the face of these undermining factors? If this happens, any seeming disparity in the magnitude of HIV-associated OIs and the benefits of HAART between HICs and LMICs may inadvertently be widened in the era of HAART.
Acknowledgements
Prof. Brian van Wyk of the School of Public Health, University of the Western Cape (UWC) South Africa and Prof. Harry Hausler of the TB-HIV Care Association, Cape Town, South Africa are deeply appreciated for their mentorship.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- UNAIDS. UNAIDS Report on the Global AIDS Epidemic 2013. Geneva, Switzerland; 2013.
- Chan IS, Neaton JD, Saravolatz LD, Crane LR, Osterberger J. Frequencies of opportunistic diseases prior to death among HIV-infected persons. Community Programs for Clinical Research on AIDS. AIDS 1995;9:1145-51.
- Sharma SK, Kadhiravan T, Banga A, Goyal T, Bhatia I, Saha PK. Spectrum of clinical disease in a series of 135 hospitalised HIV-infected patients from north India. BMC Infect Dis 2004;4:52.
- Agaba PA, Digin E, Makai R, Apena L, Agbaji OO, Idoko JA, et al. Clinical characteristics and predictors of mortality inhospitalized HIV-infected Nigerians. J Infect Dev Ctries 2011;5:377-82.
- van Lettow M, Åkesson A, Martiniuk AL, Ramsay A, Chan AK, Anderson ST, et al. Six-month mortality among HIV-infected adults presenting for antiretroviral therapy with unexplained weight loss, chronic fever or chronic diarrhea in Malawi. PLoS One 2012;7:e48856.
- Rajagopalan N, Suchitra JB, Shet A, Khan ZK, Martin-Garcia J, Nonnemacher MR, et al. Mortality among HIV-infected patients in resource limited settings: A case controlled analysis of inpatients at a community care center. Am J Infect Dis 2009;5:219-224.
- Inverarity D, Bradshaw Q, Wright P, Grant A. The spectrum of HIV-related disease in rural Central Thailand. Southeast Asian J Trop Med Public Health 2002;33:822-31.
- Sun HY, Chen MY, Hsieh SM, Sheng WH, Chang SY, Hsiao CF, et al. Changes in the clinical spectrum of opportunistic illnesses in persons with HIV infection in Taiwan in the era of highly active antiretroviral therapy. Jpn J Infect Dis 2006;59:311-6.
- De Beaudrap P, Etard JF, Diouf A, Ndiaye I, Ndèye GF, Sow PS, et al. Incidence and determinants of new AIDS-definingillnesses after HAART initiation in a Senegalese cohort. BMC Infect Dis 2010;10:179.
- Srirangaraj S, Venkatesha D. Opportunistic infections in relation to antiretroviral status among AIDS patients from south India. Indian J Med Microbiol 2011;29:395-400.
- Schiesari A, Galisteu KJ, Cardoso LV, Schiesari VM, Furini AA, Rossit AR, et al. Epidemiological patterns of AIDS in a reference center from Catanduva, São Paulo State, Brazil. Open J Med Microbiol 2012;2:47-53.
- Brodt HR, Kamps BS, Gute P, Knupp B, Staszewski S, Helm EB. Changing incidence of AIDS-defining illnesses in the era of antiretroviral combination therapy. AIDS 1997;11:1731-8.
- Chaisson RE, Gallant JE, Keruly JC, Moore RD. Impact of opportunistic disease on survival in patients with HIV infection. AIDS 1998;12:29-33.
- d’Arminio Monforte A, Sabin CA, Phillips A, Sterne J, May M, Justice A, et al. The changing incidence of AIDS events in patients receiving highly active antiretroviral therapy. Arch Intern Med 2005;165:416-23.
- Buchacz K, Baker RK, Palella FJ Jr., Chmiel JS, Lichtenstein KA, Novak RM, et al. AIDS-defining opportunistic illnesses in US patients, 1994-2007: A cohort study. AIDS 2010;24:1549-59.
- Ledergerber B, Egger M, Erard V, Weber R, Hirschel B, Furrer H, et al. AIDS-related opportunistic illnesses occurring after initiation of potent antiretroviral therapy: The Swiss HIV cohort study. JAMA 1999;282:2220-6.
- Mocroft A, Sabin CA, Youle M, Madge S, Tyrer M, Devereux H, et al. Changes in AIDS-defining illnesses in a London clinic,1987-1998. J Acquir Immune Defic Syndr 1999;21:401-7.
- Rojanawiwat A, Tsuchiya N, Pathipvanich P, Pumpradit W, Schmidt WP, Honda S, et al. Impact of the national access to antiretroviral program on the incidence of opportunistic infections in Thailand. Int Health 2011;3:101-7.
- WHO/UNAIDS/UNICEF. Global update on HIV treatment : Results, impact and opportunities. Geneva, Switzerland; 2013.
- Palella FJ Jr., Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV outpatient study investigators. N Engl J Med 1998;338:853-60.
- Bhowmik A, Bhandari S, De R, Guha SK. Predictors of mortality among HIV-infected patients initiating anti retroviral therapy at a tertiary care hospital in Eastern India. Asian Pac J Trop Med 2012;5:986-90.
- World Bank. Country Classification by Income Groups. World Bank; 2013. Available from: http://www.data.worldbank. org/about/country-classifications/country-and-lending-groups. [Last accessed on 2013 Dec 15].
- Detels R, Muñoz A, McFarlane G, Kingsley LA, Margolick JB, Giorgi J, et al. Effectiveness of potent antiretroviral therapy on time to AIDS and death in men with known HIV infection duration. Multicenter AIDS cohort study investigators. JAMA 1998;280:1497-503.
- Centers for Disease Control (CDC). Revision of the CDC surveillance case definition for acquired immunodeficiency syndrome. Council of state and territorial epidemiologists; AIDS program, center for infectious diseases. MMWR Suppl 1987;36:1S-15S.
- World Health Organization. Proposed ‘‘World Health Organization staging system for HIV infection and disease’’: Preliminary testing by an international collaborative cross-sectional study. The WHO International Collaborating Group for the Study of the WHO Staging System. AIDS 1993;7:711–718.
- Centers for Disease Control and Prevention. 1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Morb Mortal Wkly Rep 1992;41(RR-17):1–19.
- World Health Organization. Interim WHO clinical staging of HIV/AIDS and HIV/AIDS case definition for surveillance. World Health Organization 2005. www.who.int/hiv/pub/ guidelines/clinicalstaging.pdf. Accessed November 2, 2014.
- Finkelstein DM, Williams PL, Molenberghs G, Feinberg J, Powderly WG, Kahn J, et al. Patterns of opportunistic infections in patients with HIV infection. J Acquir Immune Defic Syndr Hum Retrovirol 1996;12:38-45.
- Kaplan JE, Hanson D, Dworkin MS, Frederick T, Bertolli J, Lindegren ML, et al. Epidemiology of human immunodeficiency virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clin Infect Dis 2000;30 Suppl 1:S5-14.
- Charurat M, Blattner W, Hershow R, Buck A, Zorrilla CD, Watts DH, et al. Changing trends in clinical AIDS presentations and survival among HIV-1-infected women. J Womens Health (Larchmt) 2004;13:719-30.
- Cain LE, Cole SR, Greenland S, Brown TT, Chmiel JS,Kingsley L, et al. Effect of highly active antiretroviral therapy on incident AIDS using calendar period as an instrumental variable. Am J Epidemiol 2009;169:1124-32.
- Forrest DM, Seminari E, Hogg RS, Yip B, Raboud J, Lawson L, et al. The incidence and spectrum of AIDS-defining illnessesin persons treated with antiretroviral drugs. Clin Infect Dis 1998;27:1379-85.
- San-Andrés FJ, Rubio R, Castilla J, Pulido F, Palao G, de Pedro I, et al. Incidence of acquired immunodeficiency syndrome-associated opportunistic diseases and the effect of treatment on a cohort of 1115 patients infected with human immunodeficiency virus, 1989-1997. Clin Infect Dis 2003;36:1177-85.
- Mocroft A, Katlama C, Johnson AM, Pradier C, Antunes F, Mulcahy F, et al. AIDS across Europe, 1994-98: The EuroSIDA study. Lancet 2000;356:291-6.
- Babiker A, Darbyshire J, Pezzotti P, Porter K, Rezza G, Walker SA, et al. Changes over calendar time in the risk of specific first AIDS-defining events following HIV seroconversion, adjusting for competing risks. Int J Epidemiol 2002;31:951-8.
- Ghate M, Deshpande S, Tripathy S, Nene M, Gedam P, Godbole S, et al. Incidence of common opportunistic infections in HIV-infected individuals in Pune, India: Analysis by stages of immunosuppression represented by CD4 counts. Int J Infect Dis 2009;13:e1-8.
- Gadelha AJ, Accacio N, Costa RL, Galhardo MC, Cotrim MR, de Souza RV, et al. Morbidity and survival in advanced AIDS in Rio de Janeiro, Brazil. Rev Inst Med Trop Sao Paulo 2002;44:179-86.
- Fonseca LA, Reingold AL, Casseb JR, Brigido LF, Duarte AJ. AIDS incidence and survival in a hospital-based cohort of asymptomatic HIV seropositive patients in São Paulo, Brazil. Int J Epidemiol 1999;28:1156-60.
- Seyler C, Messou E, Gabillard D, Inwoley A, Alioum A, Anglaret X. Morbidity before and after HAART initiation in sub-Saharan African HIV-infected adults: A recurrent event analysis. AIDS Res Hum Retroviruses 2007;23:1338-47.
- Badri M, Maartens G, Bekker LG, Wood R. The spectrum and prognosis of AIDS-defining illnesses in Cape Town. South Afr J HIV Med 2005;6:11-6.
- Hanna DB, Gupta LS, Jones LE, Thompson DM, Kellerman SE, Sackoff JE. AIDS-defining opportunistic illnesses in the HAART era in New York city. AIDS Care 2007;19:264-72.
- Chakravarty J, Mehta H, Parekh A, Attili SV, Agrawal NR, Singh SP, et al. Study on clinico-epidemiological profile of HIV patients in Eastern India. J Assoc Physicians India 2006;54:854-7.
- Saha K, Firdaus R, Santra P, Pal J, Roy A, Bhattacharya MK, et al. Recent pattern of Co-infection amongst HIV seropositiveindividuals in tertiary care hospital, Kolkata. Virol J 2011;8:116.
- Tansuphasawadikul S, Amornkul PN, Tanchanpong C, Limpakarnjanarat K, Kaewkungwal J, Likanonsakul S, et al. Clinical presentation of hospitalized adult patients with HIV infection and AIDS in Bangkok, Thailand. J Acquir Immune Defic Syndr 1999;21:326-32.
- Naba MR, Kanafani ZA, Awar GN, Kanj SS. Profile of opportunistic infections in HIV-infected patients at a tertiary care center in Lebanon. J Infect Public Health 2010;3:130-3.
- Corey D, Kim HW, Salazar R, Gutierrez L, Sanchez J, Tabet SR. The natural history of untreated HIV infection in Lima, Peru: Implications for clinical trial endpoints for HIV vaccines. Hum Vaccin 2005;1:160-4.
- Sunpath H, Edwin C, Chelin N, Nadesan S, Maharaj R, Moosa Y, et al. Operationalizing early antiretroviral therapy in HIV-infected in-patients with opportunistic infections including tuberculosis. Int J Tuberc Lung Dis 2012;16:917-23.
- Saidu AS, Bunza MD, Abubakar U, Adamu T, Ladan MJ, Fana SA. A survey of opportunistic infections in HIV seropositive patients attending major hospitals of Kebbi State, Nigeria. Bayero J Pure Appl Sci 2009;2:70-4.
- Daniyam CA, Iroezindu MO, Shehu N, Essien M, Sati AK, Agaba EI. Characteristics of HIV/AIDS patients presenting late at a teaching hospital in Nigeria. J Med Trop 2011;13:68-71.
- Corey DM, Kim HW, Salazar R, Illescas R, Villena J, Gutierrez L, et al. Brief report: Effectiveness of combination antiretroviral therapy on survival and opportunistic infections in a developing world setting: An observational cohort study. J Acquir Immune Defic Syndr 2007;44:451-5.
- Moore RD, Chaisson RE. Natural history of opportunistic disease in an HIV-infected urban clinical cohort. Ann Intern Med 1996;124:633-42.
- Jougla E, Péquignot F, Carbon C, Pavillon G, Mireille EB, Bourdais JP, et al. AIDS-related conditions: Study of a representative sample of 1203 patients deceased in 1992 in France. Int J Epidemiol 1996;25:190-7.
- Bonnet F, Lewden C, May T, Heripret L, Jougla E, Bevilacqua S, et al. Opportunistic infections as causes of death in HIV-infected patients in the HAART era in France. Scand J Infect Dis 2005;37:482-7.
- Yazdanpanah Y, Chêne G, Losina E, Goldie SJ, Merchadou LD, Alfandari S, et al. Incidence of primary opportunistic infections in two human immunodeficiency virus-infected French clinical cohorts. Int J Epidemiol 2001;30:864-71.
- Volkow P, Ponce de León S, Calva J, Ruiz-Palacios G, Mohar A. Transfusion associated AIDS in Mexico. Clinical spectrum, conditional latency distribution, and survival. Rev Invest Clin 1993;45:133-8.
- Moreira Júnior ED, Silva N, Brites C, Carvalho EM, Bina JC, Badaro R, et al. Characteristics of the acquired immunodeficiency syndrome in Brazil. Am J Trop Med Hyg 1993;48:687-92.
- Nobre V, Braga E, Rayes A, Serufo JC, Godoy P, Nunes N, et al. Opportunistic infections in patients with AIDS admittedto an university hospital of the Southeast of Brazil. Rev Inst Med Trop Sao Paulo 2003;45:69-74.
- Singh A, Bairy I, Shivananda PG. Spectrum of opportunistic infections in AIDS cases. Indian J Med Sci 2003;57:16-21.
- Takalkar AA, Saiprasad GS, Prasad VG, Madhekar NS. Study of opportunistic infections in HIV seropositive patients admitted to community care centre (CCC), KIMS Narketpally. Biomed Res 2012;23:139-42.
- PatelSD, KinariwalaDM, JavadekarTB. Clinico-microbiological study of opportunistic infection in HIV seropositive patients. Indian J Sex Transm Dis 2011;32:90-3.
- Chakraborty N, Mukherjee A, Santra S, Sarkar RN, Banerjee D, Guha SK, et al. Current trends of opportunistic infections among HIV-seropositive patients from Eastern India. Jpn J Infect Dis 2008;61:49-53.
- Goud TG, Ramesh K. Opportunistic infections among HIV patients attending tertiary care hospital, Karnataka, India. Int J Curr Microbiol Appl Sci 2014;3:824-9.
- Madkar SS, Vankudre AJ, Nilekar SL. Spectrum of opportunistic infections in HIV-AIDS patients. Indian J Community Health 2012;24:184-7.
- Nissapatorn V, Lee C, Fatt QK, Abdullah KA. AIDS-related opportunistic infections in Hospital Kuala Lumpur. Jpn J Infect Dis 2003;56:187-92.
- Kong BN, Harwell JI, Suos P, Lynen L, Mohiuddin S, Reinert S, et al. Opportunistic infections and HIV clinical disease stage among patients presenting for care in Phnom Penh, Cambodia. Southeast Asian J Trop Med Public Health 2007;38:62-8.
- Thongcharoen P, Vithayasai P, Vithayasai V. Opportunistic infections in AIDS/HIV-infected patients in Thailand. Thai AIDS J 1992;4:117-22.
- Xiao J, Gao G, Li Y, Zhang W, Tian Y, Huang Y, et al. Spectrums of opportunistic infections and malignancies in HIV-infected patients in tertiary care hospital, China. PLoS One 2013;8:e75915.
- Cheong I, Lim A, Lee C, Ibrahim Z, Sarvanathan K. Epidemiology and clinical characteristics of HIV-infected patients in Kuala Lumpur. Med J Malaysia 1997;52:313-7.
- Sharma S, Dhungana GP, Pokhrel BM, Rijal BP. Opportunistic infections in relation to CD4 level among HIV seropositive patients from central Nepal. Nepal Med Coll J 2010;12:1-4.
- Dhungel BA, Dhungel KU, Easow JM, Singh YI. Opportunistic infection among HIV seropositive cases in Manipal Teaching Hospital, Pokhara, Nepal. Kathmandu Univ Med J (KUMJ) 2008;6:335-9.
- Wong CS, Lo FA, Cavailler P, Ng OT, Lee CC, Leo YS, et al. Causes of death in hospitalised HIV-infected patients at a national referral centre in Singapore: A retrospective review from 2008 to 2010. Ann Acad Med Singapore 2012;41:571-6.
- Tayler-Smith K, Zachariah R, Manzi M, Kizito W, Vandenbulcke A, Dunkley S, et al. Demographic characteristics and opportunistic diseases associated with attrition during preparation for antiretroviral therapy in primary health centres in Kibera, Kenya. Trop Med Int Health 2011;16:579-84.
- Arthur G, Nduba VN, Kariuki SM, Kimari J, Bhatt SM, Gilks CF. Trends in bloodstream infections among human immunodeficiency virus-infected adults admitted to a hospital in Nairobi, Kenya, during the last decade. Clin Infect Dis 2001;33:248-56.
- Bedell RA, Anderson ST, van Lettow M, Akesson A, Corbett EL, Kumwenda M, et al. High prevalence of tuberculosis and serious bloodstream infections in ambulatory individuals presenting for antiretroviral therapy in Malawi. PLoS One 2012;7:e39347.
- Lucas SB, Hounnou A, Peacock C, Beaumel A, Djomand G, N’Gbichi JM, et al. The mortality and pathology of HIV infection in a west African city. AIDS 1993;7:1569-79.
- Grant AD, Sidibé K, Domoua K, Bonard D, Sylla-Koko F, Dosso M, et al. Spectrum of disease among HIV-infected adults hospitalised in a respiratory medicine unit in Abidjan, Côte d’Ivoire. Int J Tuberc Lung Dis 1998;2:926-34.
- Attia A, Huët C, Anglaret X, Toure S, Ouassa T, Gourvellec G, et al. HIV-1-related morbidity in adults, Abidjan, Côted’Ivoire: A nidus for bacterial diseases. J Acquir Immune Defic Syndr 2001;28:478-86.
- Anglaret X, Minga A, Gabillard D, Ouassa T, Messou E, Morris B, et al. AIDS and non-AIDS morbidity and mortality across the spectrum of CD4 cell counts in HIV-infected adults before starting antiretroviral therapy in Cote d’Ivoire. Clin Infect Dis 2012;54:714-23.
- Atzori C, Bruno A, Chichino G, Gatti S, Scaglia M. Pneumocystis carinii pneumonia and tuberculosis in Tanzanian patientsinfected with HIV. Trans R Soc Trop Med Hyg 1993;87:55-6.
- Kassa E, Rinke de Wit TF, Hailu E, Girma M, Messele T, Mariam HG, et al. Evaluation of the World Health Organization staging system for HIV infection and disease in Ethiopia: Association between clinical stages and laboratory markers. AIDS 1999;13:381-9.
- Beshah G. Study of Prevalence of Opportunistic Infections Among HIV/AIDS Patients in Addis Ababa Public Hospitals. Thesis Submitted to School of Graduate Studies of Addis Ababa University in Partial Fulfillment of the Requirement for the Degree of Master of Public Health; May, 2011.
- Wood R, O’Keefe EA, Maartens G. The changing pattern of transmission and clinical presentation of HIV infection in the Western Cape region of South Africa (1984-1995). South Afr J Epidemiol Infect 1996;11:96-8.
- Hung CC, Chang SC. Impact of highly active antiretroviral therapy on incidence and management of human immunodeficiency virus-related opportunistic infections. J Antimicrob Chemother 2004;54:849-53.
- Seyler C, Toure S, Messou E, Bonard D, Gabillard D, Anglaret X. Risk factors for active tuberculosis after antiretroviral treatment initiation in Abidjan. Am J Respir Crit Care Med 2005;172:123-7.
- Zhou J, Paton NI, Ditangco R. AIDS-defining illness diagnosed within 90 days after starting highly active antiretroviral therapy among patients from the TREAT Asia HIV observational database. Int J STD AIDS 2007;18:446-52.
- Ives NJ, Gazzard BG, Easterbrook PJ. The changing pattern of AIDS-defining illnesses with the introduction of highly active antiretroviral therapy (HAART) in a London clinic. J Infect 2001;42:134-9.
- WHO. Retention in HIV Programmes. Defining the Challenges and Identifying the Solutions. Meeting Report 13-15 September 2011. Geneva, Switzerland; 2011.
- Marks G, Gardner LI, Craw J, Crepaz N. Entry and retention in medical care among HIV-diagnosed persons: A meta-analysis. AIDS 2010;24:2665-78.
- Aghaizu A, Brown A, Nardone A, Gill O, Delpech V. HIV in the United Kingdom: 2013 Report. Available from: http:// www.gov.uk/phe. [Last accessed on 2015 Oct 09].
- Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: A systematic review. PLoS Med 2007;4:e298.
- Massaquoi M, Zachariah R, Manzi M, Pasulani O, Misindi D, Mwagomba B, et al. Patient retention and attrition on antiretroviral treatment at district level in rural Malawi. Trans R Soc Trop Med Hyg 2009;103:594-600.
- Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007-2009: Systematic review. Trop Med Int Health 2010;15 Suppl 1:1-15.
- Geng EH, Nash D, Kambugu A, Zhang Y, Braitstein P, Christopoulos KA, et al. Retention in care among HIV-infected patients in resource-limited settings: Emerging insights and new directions. Curr HIV/AIDS Rep 2010;7:234-44.
- Muwanga A, Easterbrook PJ, Schaefer P, Wandera M, Okello D, Castelnuovo B, et al. Losses to Follow-up in a Large ART Program in Uganda. 15th Conference on Retroviruses and Opportunistic Infections; Boston, MA; 3-6 February, 2008.
- Tweya H, Gareta D, Chagwera F, Ben-Smith A, Mwenyemasi J, Chiputula F, et al. Early active follow-up of patients on antiretroviral therapy (ART) who are lost to follow-up: The ‘Back-to-Care’ project in Lilongwe, Malawi. Trop Med Int Health 2010;15 Suppl 1:82-9.
- Iroezindu MO, Ofondu EO, Hausler H, Wyk BV. Prevalence and risk factors for opportunistic infections in HIV patients receiving antiretroviral therapy in a resource-limited setting in Nigeria. J AIDS Clin Res 2013;S3:002.
- Manosuthi W, Chaovavanich A, Tansuphaswadikul S, Prasithsirikul W, Inthong Y, Chottanapund S, et al. Incidence and risk factors of major opportunistic infections after initiation of antiretroviral therapy among advanced HIV-infected patients in a resource-limited setting. J Infect 2007;55:464-9.
- Schoffelen AF, van Lelyveld SF, Barth RE, Gras L, de Wolf F, Netea MG, et al. Lower incidence of Pneumocystis jirovecii pneumonia among Africans in the Netherlands host or environmental factors? AIDS 2013;27:1179-84.
- Komati S, Shaw PA, Stubbs N, Mathibedi MJ, Malan L, Sangweni P, et al. Tuberculosis risk factors and mortality for HIV-infected persons receiving antiretroviral therapy in South Africa. AIDS 2010;24:1849-55.
- Lawn SD, Badri M, Wood R. Tuberculosis among HIV-infected patients receiving HAART: Long term incidence and risk factors in a South African cohort. AIDS 2005;19:2109-16.
- Dore GJ, Li Y, McDonald A, Kaldor JM. Spectrum of AIDS-defining illnesses in Australia, 1992 to 1998: Influence of country/region of birth. J Acquir Immune Defic Syndr 2001;26:283-90.
- Del Amo J, Petruckevitch A, Phillips AN, Johnson AM, Stephenson JM, Desmond N, et al. Spectrum of disease in Africans with AIDS in London. AIDS 1996;10:1563-9.
- Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: A cohort study. Lancet 2002; 359: 2059–64.
- Paula AA, Falcão MC, Pacheco AG. Metabolic syndrome in HIV-infected individuals: Underlying mechanisms and epidemiological aspects. AIDS Res Ther 2013;10:32.
- Dau B, Holodniy M. The relationship between HIV infection and cardiovascular disease. Curr Cardiol Rev 2008;4:203-18.
- Brooks JT, Kaplan JE, Holmes KK, Benson C, Pau A, Masur H. HIV-associated opportunistic infections – Going, going, but not gone: The continued need for prevention and treatment guidelines. Clin Infect Dis 2009;48:609-11.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.