Distribution of ABO Blood Groups and Rhesus (Rh) System in Glucose-6-Phosphate Dehydrogenase (G6PD) Deficient New-borns: A Regional Study in Iran
2 Neonatal Research Center, Faculty of Medical Sciences, Mashhad, Iran, Email: saeedeh452@gmail.com
3 Students’ Research Committee, Islamic Azad University, Tehran Medical Sciences Branch, Iran, Email: bayesh123@gmail.com
4 Pediatric Hematologist and Oncologist, Department of Pediatrics, Islamic Azad University, Tehran Medical Sciences Branch, Iran
Citation: Eslami ST, et al. Distribution of ABO Blood Groups and Rhesus (Rh) System in Glucose-6-Phosphate Dehydrogenase (G6PD) Deficient New-Borns: A Regional Study in Iran. Ann Med Health Sci Res. 2018;8:262-265
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: Glucose-6- phosphate dehydrogenase (G6PD) deficiency is a common inherited disorder in Iran and may cause neonatal jaundice in some conditions. This study aimed to evaluate the distribution of ABO blood groups and Rh in G6PD deficient newborns in Iran, Mashhad. Materials and Methods: This case-control multi central study was conducted on 150 icteric newborns who admitted to the NICU of educational hospitals in North-East state of Iran, Mashhad. G6PD deficiency was evaluated and case and control groups were considered of 50 icteric newborns with G6PD deficiency and 100 icteric newborns with normal levels of enzyme. Distribution of ABO blood groups and Rh were evaluated in G6PD deficient newborns and compared to controls. The prevalence of hemolysis was compared in two groups as well. Results: Prevalence of hemolysis was 22% in case group and 19% in controls. There was no significant relationship between G6PD deficiency and hemolysis (p>0.05). Distribution of blood group A was higher in case group compared to control group (68% vs. 23% respectively), the difference was significant (Z=2.55, p=0.0054). Distribution of positive Rh blood group was higher in case group compared to control group (95% vs. 85% respectively) and the differences were not significant (p>0.05). Conclusions: There was a significant relationship between distributions of blood group A in G6PD deficient newborns.
Keywords
ABO blood-group system; Rhesus Blood Group System; G6PD deficiency; Hyperbilirubinemia; NeonatalIntroduction
G6PD deficiency is the most common human enzyme deficiency in the world. It is an X-linked, hereditary genetic defect which affects an estimated 400 million people, mostly African, Mediterranean and far-eastern populations. [1,2] According to the report of WHO, the overall incidence of G6PD deficiency among the Iranian population was 10−14.9%. [3] A meta-analysis regard G6PD deficiency showed its incidence in Iran was between 2.1 to 7.6%. [4] This enzyme deficiency is very prevalent in individuals of Africa, America, Mediterranean, and East Asia. In India the incidence of G6PD deficiency has been variably reported as 0–37% in different castes and communities with higher incidence in north and west India (15%) as compared to south India (1-2%). [5] In Kuwait, G6PD deficiency was found to be 22.3% in newborns and 20.4% in adults with a mean average of 21.3%. [6]
Neonatal jaundice and hemolytic anemia are two major outcomes associated with G6PD deficiency. A high level of unconjugated bilirubin is a strong neurotoxic agent. Kernicterus is a neurologic syndrome resulting from deposition of unconjugated bilirubin in the basal ganglia and brain stem nuclei which may cause severe neurological complications and even death in some populations. [7-9] Many studies have been performed to decrease bilirubin level with phototherapy and herbal extracts. [10] Hemolytic anemia results from stress factors such as certain oxidative drugs, infections or fava beans. G6PD deficiency is also known as ‘fauvism’, a term used to describe acute hemolytic anemia after the consumption broad beans (Vicia faba) that is a common food in Iran. [11,12]
Numerous associations have been made between particular ABO phenotypes and an increased susceptibility to diseases. For example, a peptic ulcer (PU) is significantly more common in O blood group individuals [13] and gastric or pancreas cancers are more common in A blood group individuals. [14] Therefore, we aimed to determine distribution of ABO blood groups and Rh in G6PD deficient newborns and to compare the results in newborns with normal levels of the enzyme in Iran. The prevalence of hemolysis was compared in two groups as well.
Material and Methods
This case-control multi central prospective study included 150 newborns with hyperbilirubinemia who were admitted to NICU of educational hospitals related to Azad University from 2012 to 2014 in North-East state of Iran, Mashhad. Inclusion criteria were healthy term newborns with hyperbilirubinemia. Newborns with any other disease, premature and those aged older than 28 days, were excluded. According to the mean related prevalence,
α = 5% = 1.96 and β = 20% = 0.84
and
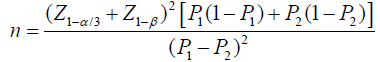
Sample size was considered about 53 newborns that met inclusion criteria and enrolled in the study as case group and 102 newborns considered as control group. Three newborns in case group and two newborns in control group refused the study. Therefore 50 and 100 newborns remained as case and control groups respectively. The method of sampling was non-probability of easy sampling.
Data collection form was designed for the study. Clinical jaundice was determined by yellowish color of sclera, mucosa and skin. A detailed history and clinical examination was performed for the subjects and demographic data and birth history was recorded as well.
The population of interest was screened for the quantitative measurement of G6PD activity by enzymatic colorimetric assay. Newborns with value <6.4 U/g Hb were considered as G6PD deficient. Case group included 50 newborns with G6PD deficiency and 100 newborns without enzyme deficiency were considered as control group. Distribution of ABO blood groups and Rh was determined in G6PD deficient newborns and compared to newborns with normal levels of the enzyme. The prevalence of hemolysis was compared in two groups as well. Hemolysis was considered as meeting any of the following criteria: hematocrit (Hct) <40% in the absence of acute blood loss, reticulocyte count >5% in newborns aged birth to 5 days and >1% in newborns older than 5 days and positive Coombs (considered dilution studies if there is clinically confident of hemolytic process with negative coombs test). Variables as age, gender, weight, height, maternal age, number and delivery type, Apgar score in 1 and 5 minutes Hct, reticulocyte and bilirubin were determined as well.
Ethical considerations
This study was conducted according to the guidelines of Helsinki, the Guidelines for the Ethical Conduct of Medical Research Involving Children, revised by the Royal College of Pediatrics and Child Health: Ethics Advisory Committee. We considered Committee on Publication Ethics (COPE) guidelines as well. There was no moral inconsideration, and all the cooperators and the parents were well explained about the study method and we received informed consent and ethical approval.
The data was analyzed. For comparison of results, T-Test was used and for the quality variants, Fisher and Chi-Square tests were used with P-value <0.05 through SPSS software.
Results
150 newborns met the enrolment criteria and participated in the study. Case group included 50 icteric newborns with G6PD deficiency which 36 (72%) were males and 14 (28%) were females [Table 1].
| Case group (G6PD deficient) | Gender N (%) | Male/Female Ratio | Total | p-value | |
| Males | Females | ||||
| 36 (72) | 14 (28) | 2.5:1 | 50 (33.3) | p=0.0009 | |
Table 1: Proportion of males and females in the case group (G6PD deficient).
In case group, there was significant statistical difference between male and female infants that more than 50% of G6PD deficient newborns were males (p=0.0009). Control group included 100 newborns, which 57 (57%) were males and 43 (43%) were females.
The mean age of newborns was 5.80 ± 0.34 and 5.54 ± 0.29 days in case and control groups respectively. The mean weight of newborns was 2947.4 ± 68.91 gr and 3108.65 ± 56.85 gr in case and control groups respectively, and the mean height of newborns was 49.14 ± 0.49 in case group and 49.55 ± 0.26 cm in controls. There were no significant differences between two groups by T test in regard to the mean age, weight and height, maternal age, number and delivery type, and Apgar score in 1 and 5 minutes (p>0.05).
The mean Hct was lower in case group (42.35 ± 1.27 vs. 45.70 ± 0.75), but the difference was not significant (p>0.05).
Average of total bilirubin level was 21.32 ± 0.96 mg/dl in case group and 21.41 ± 3.16 mg/dl in control group, with no significant difference between two groups. The mean indirect bilirubin level was 20.31 ± 0.94 mg/dl and 20.69 ± 3.15 mg/dl in case and control groups respectively, with no significant difference between two groups (p>0.05). The mean reticulocyte count was higher in case group (2.42 ± 0.30 vs. 1.97 ± 0.17) but with no significant difference between two groups (p>0.05). In this study, coombs test was 100% negative in both case and control groups.
Table 2 shows hemolysis, ABO blood groups and Rh distribution in case and control groups.
| Variables | (G6PD deficient) | Control | Total | p-value | |
|---|---|---|---|---|---|
| N (%) | |||||
| Hemolysis | Hemolysis | 11 (22) | 19 (19) | 30 (20) | p>0.05 |
| Non hemolysis | 39 (78) | 81 (81) | 120 (80) | ||
| Total | 50 (100) | 100 (100) | 150 (100) | ||
| Blood groups | A | 34 (68) | 23 (23) | 57 (38) | p=0.0054 |
| B | 4 (8) | 30 (30) | 34 (22) | p>0.05 | |
| AB | 2 (4) | 10 (10) | 12 (8) | p>0.05 | |
| O | 10 (20) | 37 (37) | 47 (31) | p>0.05 | |
| Total | 50 (100) | 100 (100) | 150 (100) | ||
| RH | Negative | 5 (10) | 15 (15) | 20 (13.3) | p>0.05 |
| Positive | 45 (90) | 85 (85) | 130 (86.7) | p>0.05 | |
| Total | 50 (100) | 100 (100) | 150 (100) | ||
Table 2: Hemolysis, ABO blood groups and Rh distribution in case (G6PD deficient) and control groups.
Comparing hemolysis between case and control groups revealed that in case group, 11 (22%) neonates had hemolysis and 39 (78%) neonates had not hemolysis. In control group, 19 (19%) of neonates had hemolysis and 81 patients (81%) were not hemolytic. There was no significant relationship between existence of hemolysis and enzyme deficiency by Fisher Exact Test (p>0.05). In evaluation of ABO blood groups in G6PD deficient newborns, the distribution was as follows: 34 (68%) of newborns in case and 23 (23%) in control group had A blood group. Although distribution of A blood group was higher in case group compared to control group (68% vs. 23% respectively), the difference was significant (p<0.05) [Table 2]. (Z=2.55, p=0.0054). Distribution of B blood group was 4 (8%) in case and 30 (30%) in control group of newborns. About AB blood group, 2 (4%) and 10 (10%) of newborns had AB blood group in case and control groups, respectively. There was no significant association between the AB blood group and G6PD deficiency (p>0.05). Distribution of O blood group was 10 (20%) in case and 37 (37%) control group. In total analyses, there was no significant association between the distribution of ABO blood groups and G6PD deficient newborns by Fisher Exact Test (p>0.05).
In distribution of Rh blood group in case and control groups, 45 (90%) newborns had positive and 5 (10%) had negative Rh blood group in case group. 85 (85%) newborns had positive and 15 (15%) newborns had negative Rh blood group in control group. Although distribution of positive Rh blood group individuals was higher in case group compared to control group (95% vs. 85%, respectively).
In total analyses, there was no significant association between the distributions of Rh blood group in G6PD deficient newborns by Fisher Exact Test (p>0.05).
Discussion
As expected, G6PD deficiency was significantly more in male newborns in present study (p=0.0009). In similar studies in Iran, the male to female ratio in newborns who were found to have G6PD deficiency was 5 to 1, [15] 5.5 to 1. [16] and 3 to 1. [17] In present study, male to female ratio in these newborns was 2.5:1. In a similar study, the prevalence of G6PD deficiency was 11% among the children and male to female ratio was greater in non- hemolytic vs. hemolytic group so that the female share was significantly p= (p=0.004) higher in hemolytic group. [18] In present study, although mean Ht in case group was lower than controls, but there were no significant differences between mean Ht and existence of hemolysis and enzyme deficiency. In a similar study, among 12 newborns with G6PD deficiency, hemolysis was seen in 5 (41.7%) newborns and the rate of G6PD deficiency without hemolysis was 2.6%. [15] In a similar study, most of G6PD deficient newborns had non-hemolysis. [17] Nabavizadeh et al. reported that there was no evidence of higher hemolysis among newborns suffering from jaundice having G6PD deficiency compared to icteric neonates with normal enzyme activity. [4] In our study, the mean indirect bilirubin levels were similar in case and control groups. The mean reticulocyte count in case group was higher than control group, but with no significant difference (p>0.05). The mean bilirubin and hemoglobin level and also reticulocyte count was similar between case and control groups in a study in Iran. [15] In another study, hyperbilirubinemia and jaundice are approximately 3-fold higher in G6PD-deficient group than in the G6PD-normal group (51% vs. 16%). [19]
Numerous associations have been reported between particular ABO phenotypes and an increased or decreased susceptibility to diseases. For example, individuals with blood type O tend to have lower levels of the von Willebrand Factor (vWF) (12). Cihan et al. study revealed that there is a significant relationship between non-melanoma skin cancer and ABO/Rh factors. [20]
In all cancers, the highest frequency of blood group B (40.5%), followed by A (34.2%), and O (16.0%) and AB (9.3%) was seen. A high incidence of blood group B (37.5%) followed by A (35%) was seen in oral cancers. Among gastrointestinal (GI) tract cancers, a high frequency of blood group B (40%), followed by O (26.7%) was noted. The incidence of A blood group was significantly higher in breast cancer and lung cancer (42.4% and 50.0% respectively). [21] In patients with pancreatic cancer the incidence of blood group O (31%) was lower compared to 13.044 patients without pancreatic cancer (38%) (p=0.0005). [22] People with blood group B are at risk of developing oral cancer and women under 50 years of age with blood B group are at highest risk of developing non –squamous cell oral cancer. [23]
Blood group A is associated with increased risk and blood group O is associated with decreased risk of cancer. [24]
A study in Iran reported that the prevalence of coronary artery diseases (CAD) in blood group O is markedly higher than in all other ABO blood groups, which is in contrast with other studies done in Europe and the United. [25] A similar study in China revealed that there are no significant differences in the frequency distribution of ABO blood groups between leprosy patients and normal controls. [26]
Racial and ethnic distribution of blood groups is an important factor for predicting cancer and other disease.
As distribution of several diseases may be related to a certain blood group, we tried to determine association between ABO blood groups and Rh with G6PD deficiency. In present study distribution of A blood group was higher in case group compared to control group (68% vs. 23% respectively) and the difference was significant (p=0.0054). Distribution of positive Rh blood group was higher in case group compared to control group (95% vs. 85% respectively) and also distribution of negative Rh blood group in case group was lower than control group (10% vs. 15% respectively), but the differences were not significant. Saha et al. study pointed out that there was no association between ABO blood groups and G6PD deficiency observed in either Chinese or Malays newborns. They reported that for the Rh system there was found to be a statistically significant decrease in the frequency of genotypes containing the complex R1, in G6PD deficient subjects compared with that in normal subjects. [27] In a similar study, the distribution of ABO blood groups and also Rh negative types was similar among asymptomatic non-G6PD deficient, asymptomatic G6PD- deficient and G6PD-deficient children with hemolysis. [18]
Conclusion
More studies are needed to confirm this subject. In addition, since the test has a low cost, it is better to test for G6PD deficiency in all of the newborns, including female infants to detect its presence and to prevent its complications such as favism and oxidant drug-induced hemolysis. Breastfeeding is the best way to feed G6PD deficient baby but an important emphasis and less recommended matter is that the nursing mothers need to avoid the same things that their G6PD deficient baby needs to avoid.
Conflict of Interest
The authors disclose that they have no conflicts of interest.
REFERENCES
- Maheshwari A, Carlo WA. Digestive system disorder. In: Kleigman RM, Stanton BF, Schor NF, Geme III JW, Behraman RE, (editors). Nelson Texbook of Pediatrics. Philadelphia: Elsevier Saunders; 2011;603-612.
- Clarence W, Gowen JR. Anemia and hyperbilirubinemia. In: Marcdante KJ, Kleigman RM, Jenson HB, Behraman RE, (editors). Nelson Texbook of Pediatrics. Philadelphia: Elsevier Saunders; 2011; 247-250.
- WHO Working Group. World Map of G6PD deficiency. Bull WHO 67:601-611.
- Nabavizadeh SH, Rezaie M, Sabzali P, Barati A, Zoladl M. cohort study on hemolysis associated with G6PD deficiency in jaundice neonates. Life Sci J. 2012;9:702-705.
- Shanthala Devi AM, Helen R, Vanamala A, Chaithra V, Karuna R. Screening for G6PD Deficiency in Blood Donor Population. Indian J Hematol Blood Transfus. 2010; 26:122-123.
- Shaker Y, Onsi A, Aziz R. The frequency of glucose-6-phosphate dehydrogenase deficiency in the newborns and adults in Kuwait. Am J Hum Genet. 1966;18:609-613.
- Beutler E. G6PD deficiency. Blood. 1994; 84:3613-3636.
- Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet. 2008;371:64-74.
- Mason PJ, Bautista JM, Gilsanz F. G6PD deficiency: The genotype-phenotype association. Blood Rev. 2007;21:267-283.
- Nassirian H, Eslami ST. Effects of chichoriumintybus on bilirubin. Indian J Pediatr. 2008;75:331-333.
- Mortazavi Y, Mirzamohammadi F, Teremahi AM, Mirimoghaddam E, Vulliamy TJ. Glucose 6-phosphate deficiency in Tehran, Zanjan and SistanBalouchestan provinces: prevalence and frequency of Mediterranean variantof G6PD. Iran J Biotechnol. 2010;8:229-233.
- Bethesda DL. Blood groups and red cell antigens (MD): National Center for Biotechnology Information, USA 2005.
- Macafee AL. ABO blood groups and gastric ulcers. J med Genet. 1965;2:24-25.
- Newell GR, Gordon JE, Monlezun AP, Horwitz JS. ABO Blood groups and cancer. JNCI J Natl Cancer Inst. 1974;52:1425-1430.
- Eghbalian F, Monsef AR. Evaluation of glucose-6-phosphate dehydrogenase deficiency without hemolysis in icteric newborns. Iran J Ped. 2007;17:36-40.
- Iranpour R, Hashemipour M, Talaei SM, Soroshnia M, Amini A. Newborn screening for glucose-6-phosphate dehydrogenase deficiency in Isfahan, Iran: A quantitative assay. J Med Screen. 2008;15:62-64.
- Ahmadi AH, Ghazizadeh Z. Evaluation of glucose-6-phosphate dehydrogenase deficiency without hemolysis in icteric newborns at Mazandaran province, Iran. Pak J Biol Sci. 2008;11:1394-1397.
- Sadeghi E, Perikala VK, Haghshenas M, Jalaeian H. Hemolysis induced by glucose-6-phosphate dehydrogenase deficiency and its association with sex in children. IJMS. 2015;35:1-5.
- Abolghasemi H, Mehrani H, Amid A. An update on the prevalence of glucose-6-phosphate dehydrogenase deficiency and neonatal jaundice in Tehran neonates. Clin Biochem. 2004;37:241-244.
- Cihan YB, Baykan H, Kavuncuoglu E, Mutlu H, Kucukoglu MB, Ozyurt K, et al. Relationships between skin cancers and blood groups-link between non-melanomas and ABO/Rh factors. Asian Pac J Cancer Prev. 2013;14:4199-4193.
- Akhtar K, Mehdi G, Sherwani R, Sofi L. Relationship between various cancers and ABO blood groups – A Northern India Experience. Internet J Pathol. 2010;13:3-7.
- Rahbari NN, Bork U, Hinz U, Leo A, Kirchberg J, Koch M, et al. AB0 blood group and prognosis in patients with pancreatic cancer. BMC Cancer. 2012;12:319.
- Mortazavi H, Hajian S, Fadavi E, Sabour S, Baharvand M, Bakhtiari S. ABO blood groups in oral cancer: A first case –control study in a Defined Group of Iranian Patients. Asian Pac J Cancer Prev. 2014;15:1415-1418.
- Zhang BL, He N, Huang YB, Song FJ, Chen KX. ABO blood groups and risk of cancer: A systematic review and meta-analysis. Asian Pac J Cancer Prev. 2014;15:4643-4650.
- Sotoudeh M, Boroumand MA, Emami B, Karimi A, Soleymanzadeh M, Abbasi SH, et al. ABO Blood group and coronary artery diseases in iranian patients. awaiting coronary artery bypass graft surgery: A review of 10, 641 cases. Lab Medicine. 2009;40:528-530.
- Saha N, Wong HB, Banerjee B, Wong MO. Distribution of ABO blood groups, G6PD deficiency, and abnormal haemoglobins in leprosy. J Med Genet. 1971;8:315-316.
- Saha N, Hawkins BR, Wong HB. Distribution of ABO and Rhesus blood groups in G6PD deficient Chinese and Malay new-borns. Acta Geneticae Medicae Et Gemellologiae. 1975;24:131-135.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.