Effect of Scapular Upward Rotation Exercises on Brachial Plexus Mechano Sensitivity in Subjects with Depressed Scapular Alignment
2 Basic Sciences Department, Cairo University, Cairo, Egypt
Received: 04-Feb-2022, Manuscript No. AMHSR-22-53608; Editor assigned: 07-Feb-2022, Pre QC No. AMHSR-22-53608(PQ); Accepted Date: Feb 27, 2022 ; Reviewed: 14-Feb-2022 QC No. AMHSR-22-53608; Revised: 21-Feb-2022, Manuscript No. AMHSR-22-53608(R); Published: 27-Feb-2022, DOI: 10.54608.annalsmedical.2022.32
Citation: Al Afify DH, et al. Effect of Scapular Upward Rotation Exercises on Brachial Plexus Mechano Sensitivity in Subjects with Depressed Scapular Alignment. Ann Med Health Sci Res. 2022;12:72-81.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
The current study aimed to assess the effect of upward rotation exercises on brachial plexus mechano sensitivity in depressed scapular alignment subjects. Thirty subjects classified as depressed scapular alignment were recruited in this study through a screening test into a pre-test post-test single group experimental design. Mechano sensitivity was assessed pre-test and post-test through application of sensory and motor strength-duration curve tests on the nerve to trapezius and upper limb neuro dynamic test assessing symptoms response in terms of pain intensity and quality. All measures were performed on the scapula classified previously as depressed following the same order. All patients received intervention in the form of scapular upward rotation exercises for 6 weeks, 3 days per week. As compared to pre-treatment values, there was a significant increase in post-treatment sensory and motor Rheobase values and a significant decrease in post-treatment sensory and motor Chronaxie values (p<0.001). In the upper limb neuro dynamic test, there was a significant decrease in pain intensity of median, ulnar and radial nerves post- treatment compared with that of pre-treatment (p<0.001). There was a significant improvement in pain quality of median, ulnar and radial nerves post-treatment as compared to that of pre-treatment (p<0.001). These results suggest that scapular upward rotation exercises reduce irritability of neural tissues in subjects with scapular depression alignment.
Keywords
Depressed scapular alignment; Strength duration curve; Neuro dynamic test; Scapular upward rotation exercises
Introduction
The scapula is critical for proper shoulder joint function, both anatomically and biomechanically. Within this text, its title and abstract, the term “scapular downward rotation” equates and is interchangeably used with the term “depressed scapular alignment”. Scapular Downward Rotation (SDR) is a scapular mal-alignments characterized by allocation of scapular inferior border more medially than the superior border, scapular depression and downward sloping at the acromial end. The weight of the upper extremities, transferred through cervico-scapular muscle attachments, might exerts a prolonged compressive and/or stretching stress on the muscles acting on the cervical spine (upper trapezius and levator scapulae). [1,2] Hence, changes in scapular and clavicular alignment may affect the shoulder biomechanics through generating excessive tension at the cervico-scapular muscles (upper trapezius muscle elongation, and levator scapula stiffness), that consequently results in insufficient scapular upward rotation, and glenohumeral joint instability during arm elevation. [3,4]
Depressed scapular alignment causes nerve roots in the brachial plexus to be exposed to prolonged and/or recurrent stress or compression, sensitizing neural tissues and potentially triggering neck-arm pain symptoms. [1] The increased neural tissue mechano sensitivity observed in SDR subjects, could be attributed to the excessive stretch and/or compression acting on the brachial plexus secondary to the scapular downward rotation. [5] Neural tissue sensitization of the upper limb is assessed through the application of neuro dynamic tension tests of peripheral nerve trunks. [6] As clinical practice is currently seeking objectivity in assessment as well as treatment, we used the Strength Duration Curve (SDC) parameters to provide an objective measure for neural tissue excitability owing to scapular downward rotation alignment.
Previous studies suggests that SDR examinations include passive correction with braces and exercise programs. [7-10] To reduce upper trapezius muscular tension, a shoulder-lifting equipment was used. [7,8] To normalize muscular activation and reduce pain, a crossed brassiere straps was used. [9] Exercise programmes, in conjunction with passive correction, have been used and demonstrated being effective. A 6-weeks exercise programme including both resistive and non-resistive upper extremity workouts have proved its usefulness in restoring scapular alignment and regaining normal scapular upward rotators’ power. Variations in the shrug exercise were also helpful for increasing the upward rotation angle and muscle activation, according to Lee et al. Although passive corrective approaches and exercise programmes have been shown to help people regain muscle function, more exercise programmes need to be researched. Lee et al. have suggested that variations in the shrug exercise enhanced the scapular upward rotation angle as well as muscular activity. Despite exercise program in conjunction with passive scapular correction have shown positive effects for regaining muscular function, it cannot be over looked that further exercise programs should be proposed and investigated. The current study was designed to investigate the effect of Scapular Upward Rotation Exercises (SURE) on brachial plexus mechano sensitivity in subjects with depressed scapular alignment.
Materials and Methods
Participants
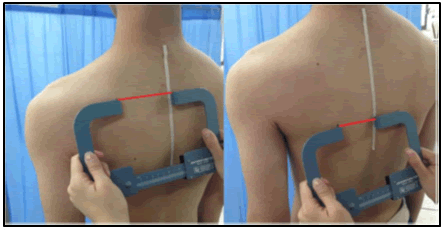
Through screening test for scapular downward rotation alignment among students of the faculty of Physical Therapy, Badr University, forty subjects met the inclusion criteria and were recruited in the current study. Subjects’ inclusion criteria in the current study, as per the literature, is the presence of scapular downward rotation (11), conformed by; visual appraisal of the scapula which show one of three main confirmative signs; 1) Inferior angle of the scapula is located closer to the spine than the root of the spine of the scapula, 2) Horizontally oriented clavicle or if the acromioclavicular joint appears by visual appraisal to be lower than sternoclavicular joint, or 3) Reduced distance between thoracic spine and vertebral border of the scapula to less than three inches through using tape measurement [Figure 1]. Any subject showing or having one of the following criteria was excluded from the study; 1) Cervical spinal fractures, 2) Neck-rotation ROM of <20º, 3) Radiating pain to one of the upper extremities, 4) Polyneuropathy, 5) Myofascial pain syndrome, 6) Fibromyalgia, 7) Thoracic scoliosis, 8) Leg length discrepancy, 9) History of unresolved cancer, 10) Diabetes mellitus, and 11) Any other scapular mal-alignment other than SDR (Ha et al., 2016). Among subjects meeting the inclusion criteria, three subjects declined, and seven missed more than two consecutive sessions and were hence excluded from the current study. Before being finally enrolled in the SURE program, all recruited subjects received an explanation of the treatment program applied and were requested to sign an informed consent. Thirty subjects with scapular downward rotation syndrome with mean age of 24.5 ± 4.27 years, were enrolled in the current study, and completed the exercise program course. The subjects in this study were four males (13.3%) and 26 females (86.7%) with 23 subjects (77%) right-handed, and 7 subjects (23%) left-handed. In all recruited subjects the dominant side was the affected side. All patients were assessed clinically pre and post-test using SDC for motor and sensory nerves of trapezius muscle and neurodynamic test for median, ulnar and radial nerves.
Methods
Assessment procedures
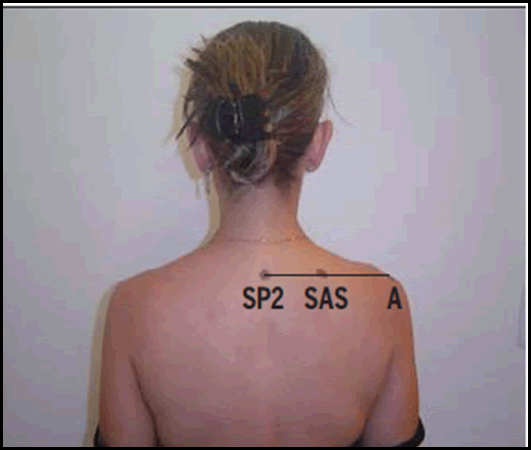
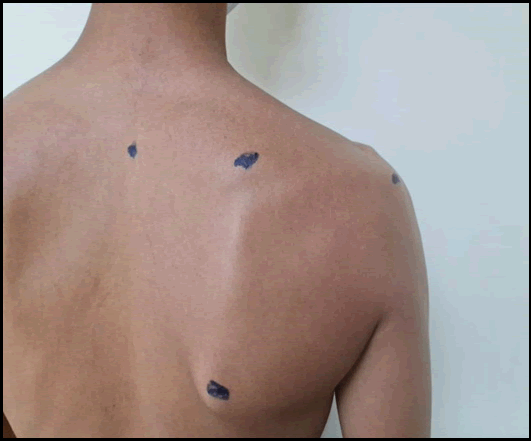
Scapular visual examination: A detailed explanation of the study and a detailed patient information sheet was given to all the subjects. Written informed consent was obtained from all the study subjects. This study was approved by the research ethics committee of faculty of physical therapy, Cairo university (P.T.REC/012/002640). It was recorded in clinical trials under no NCT04775251. The three-cardinal anatomical sitesof the scapula; Superior Angle of the Scapula (SAS), lateral border of the Acromion (A) and spinal process of the Second Thoracic vertebra (ST2), were marked with a demographic pen. Scapular alignment was categorized as: (1) “Normal”, if the “SAS” and “A” were on the same level or above “ST2” [Figure 2] and (2) “Depressed”, if “SAS” and “A” were below “ST2” [Figure 3]. [11-13] In case any other combination of positions of these reference points wasobserved, the subject wasexcluded from the study for mismatching the inclusion criteria. Validation of palpation of scapular and thoracic spinal reference points had been previously demonstrated by Lewis et al. Intra-rater and inter-rater reliability of palpation of scapular and thoracic landmarks for determining scapular position have been proven. [14,15]
Upper Limb Neurodynamic Test (ULNT)
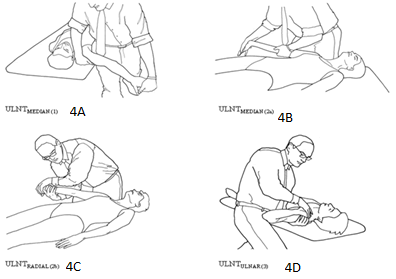
Neuro dynamic tests for the upper limb nerves consist of four distinct tests designed to evaluate the mechanosensitivity of the upper limb nerves; median, radial and ulnar nerves. Two of these tests are biased towards the median nerve (ULTT1a and ULTT1b), one for the radial nerve (ULTT2) and one for the ulnar nerve (ULTT3). These tests were performed to assess the symptoms response in terms of pain intensity and quality of pain. After explaining the procedure, measuring performance and giving all relevant instructions to the participants, the Upper Limp Neurodynamic Tests (ULTT) were performedin the same steps and standard sequencesdescribed by, involving 1) Shoulder girdle fixation, 2) Shoulder abduction, 3) Wrist extension, 4) Supination, 5) Shoulder external rotation, and 6) Elbow extension. The tests were performed only once each. As per Lohkamp and Small, (2011), the patients assumed the supine position, with no pillows, arms straight on the side of the body, and extended lower limb, as a standardized starting position [Figure 4]. For the ULTT 1a, the starting position was 90º for each of shoulder flexion, abduction and elbow flexion, forearm in supination, and maximum extension of wrist and fingers. One of the investigator’s hands prevented scapular elevation, while the other maintained finger extension and slowly executed elbow extension until the point of pain tolerance [Figure 4A]. To assume the starting position of the ULTT 1b for median nerve, the participant moved to the side of the plinth, while the investigator depressed the shoulder girdle with her thigh. The shoulder was kept in 10º of abduction, and in external rotation, elbow was extended and supinated, and maximum wrist and finger extension as well. One of the investigator’s hands ensured finger and wrist extension, while the other kept elbow extension [Figure 4B]. [16] The ULTT 2 for radial nerve was performed through depressing shoulder girdle, with shoulder joint abduction and internal rotation, extending elbow joint, pronating the forearm, and assuming wrist/finger flexion [Figure 4C]. The ULTT 3 for ulnar nerve was performed through shoulder girdle depression, shoulder abduction and external rotation, elbow flexion, forearm pronation, wrist/finger extension [Figure 4D]. [6] The participant was requested to give feedback on his/her response to the application of the ULTT. Not only the response of the test was recorded, but also, the point or phase of the test during which symptoms begin to be perceived. This point is to be called the point of symptom onset. Participant were also requested to identify the point of pain tolerance at which the symptoms are too uncomfortable to continue with the test procedure. [16,17] At the point of pain tolerance, the participant is as well requested to report pain intensity, quality and distribution of his/her symptoms. ULTT measures have been proven to have an excellent intra-rater and good inter-rater reliability in asymptomatic population, as well as acceptable precision for both intra-rater and inter-rater measurements. [18]
Symptom response: A Numerical Rating Scale (NRS, 0 no symptoms-10 the most intense pain imaginable) was used to measure the intensity of pain at the point of tolerance during the ULTT. [16,17] Quality or nature of symptoms at the point of tolerance during the ULTT, was described by the participant using one of the following descriptions: stretching, pain, tingling, pricking, numbness and burning, or a combination of several. The test-retest reliability for the NRS has been demonstrated to be moderate to high, varying from 0.67 to 0.96 and the convergent validity is 0.79 to 0.95. [17]
Measurement of Strength Duration Curve (SDC)
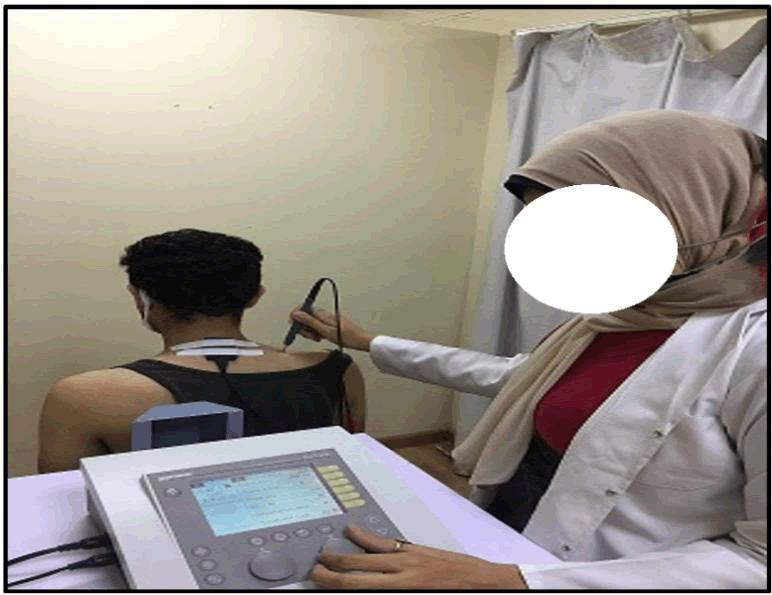
The assessment of SDC parameters for the recruited individuals was carried out with the help of a diagnostic muscle stimulator (Gymna Duo 200, Manufacturer: GymnaUniphy NV., made in: Pasweg 6 A, 3740 Bilzen, Belgium, SN:74630) using interrupted galvanic current. It utilized a unipolar direct current output with different pulse widths. Pulse width was gradually increased throughout the test starting at 0.01 msec and reaching a maximum of 300 msec. Gradually increasing intensity between 0 V and 120 V at each and every pulse width utilized, considering pulse rate as 1 pulse/s. Before current application, the skin resistance was reduced by washing and soaking in warm water and any abrasions were protected. The reference electrode was placed on the spinous process of C7 on the midline of the body and the active pen electrode was placed over the belly of the trapezius (mid-point from acromion to spinous process) [Figure 5]. For motor threshold, spinal accessory nerve (motor supply for trapezius muscle) was stimulated and current was applied using the longest stimulus first and increased until the minimum observable contraction was obtained. This was visually assessed or by palpation of the tendon. For sensory threshold, supraclavicular nerve (C3, C4) (cutaneous supply over the region of the upper trapezius muscle) was stimulated and current was applied using the longest stimulus first and intensity was increased until the minimum sensation was perceived by the individual. Sensory test depends mainly on the subject’s feedback. For both motor and sensory tests, the same procedures were used to obtain response at different pulse widths, and hence the SDC was plotted and obtained, through noting and recording the current magnitude (or voltage) at all pulse widths used. All measures were taken in the same order on the same side of the scapula that had previously been classified as depressed.
Intervention Scapular upward rotation Exercise (SURE)
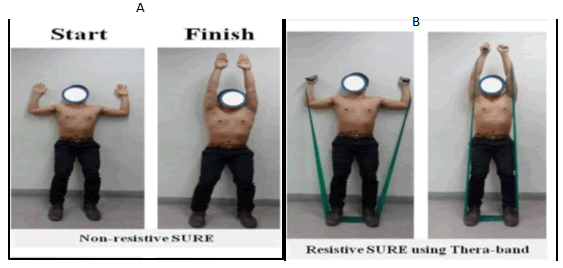
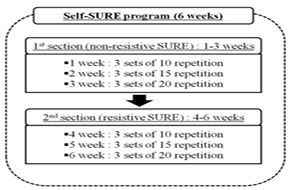
To perform the SURE, the subject was asked to stand with his/her back against the wall, with maintaining full torso contact with the wall, with feet at shoulder-width apart. The radial side of the forearm and lateral side of the arm were in contact with the wall at the starting position and throughout the performance of the exercise. The arm was positioned at 90º shoulder abduction and 90º elbow flexion. The subject was instructed to slide his/her arms up the wall, till the shoulder reached 180º of abduction. Proper performance was ensured by asking the subject to touch his/her earlobe with the shoulder, and maintain this position for 10 seconds [Figure 6]. The exercises might induce muscle fatigue, but is not expected to induce pain, and subjects were informed of so. The SURE exercise was performed as 6-weeks program, with a frequency of three days per week. The 6-weeks SURE program was divided into two sections; the first section consists of non–resistive SURE exercises during first three weeks (weeks 1-3) and the second section with resistive SURE exercises using a thera band during the next three weeks (weeks 4-6) [Figure 6]. The progression of SURE program occurs gradually through stages of the program in term of repetition, so that, the subjects were requested to perform 3 sets of 10 repetitions each during the first (non-resistive) and fourth (resistive) weeks of the program, 3 sets of 15 repetitions each during the second and fifth weeks and 3 sets of 20 repetitions each during the third and sixth weeks [Figure 7]. Thera Band tension was controlled in a low level by a concentric exercise phase to eliminate excessive activated muscle loading and joint pain. Thera Band tension level was increased by shortening the length of the thera band to within subject’s tolerance without inflicting discomfort or pain while sticking to the exercise performance instructions given by the investigator. At each session, the length of the thera band was modified. The theraband strength was selected for each subject before applying SURE by changing the thera band colour. Subjects who can execute 20 repetitions of the SURE, could change the colours and length of theraband. Subjects were requested to elongate the theraband if they felt or complained of discomfort during the resistive SURE utilizing thera band. [7,8]
Statistical Analysis
Descriptive statistics of mean, and standard deviation were conducted for expression of the subjects’ demographic characteristics of the study group. Paired t-test was conducted for comparison between pre and post treatment mean values of strength duration curve parameters. Wilcoxon Signed Ranks Test was conducted for comparison between pre and post treatment median values of pain intensity. Test of marginal homogeneity was conducted for comparison between pre and post treatment quality of pain. The level of significance for all statistical tests was set at p<0.05. All statistical measures were performed through the Statistical Package For Social Studies (SPSS) version 25 for windows.
Results
Subjects’ demographic characteristics
Thirty subjects with scapular downward rotation were recruited in this study. The mean ± SD of subjects’ age in the study group was 24.5 ± 4.27 years. The subjects in study group were four males (13.3%) and 26 females (86.7%). 23 subjects (77%) were right-handed, and 7 subjects (23%) were left-handed. The mean ± SD of subjects’ weight (Kg), height (cm), and BMI (kg/m²) were 65.41 ± 8.78 Kg, 164.86 cm ± 5.44 cm, and 24.04 kg/m² ± 2.83 kg/m². Table 1 shows the subjects’ demographic characteristics.
Effect of treatment on pain intensity and quality of pain (neuro dynamic tests)
Pain intensity significantly decreased for median, radial and ulnar nerves post-treatment compared with that of pre-treatment values (p<0.001). [Table 2] There was a significant improvement in pain quality of median, radial and ulnar nerves post-treatment compared with that of pre-treatment (p<0.001). Pain quality shifted to stretching (the least perceivable pain quality) in most of cases post-treatment [Table 3].
| Mean ± SD | Minimum | Maximum | |
|---|---|---|---|
| Age (years) | 24.5 ± 4.27 | 19 | 31 |
| Weight (kg) | 65.41 ± 8.78 | 54 | 88 |
| Height (cm) | 164.86 ± 5.44 | 156 | 176 |
| BMI (kg/m²) | 24.04 ± 2.83 | 20.08 | 30.81 |
SD: Standard Deviation |
Table 1: Descriptive statistics (mean ± SD) of subjects' demographic data (age, weight, height and BMI) of the study group.
| Median (IQR) | Z- value | p-value | |||
|---|---|---|---|---|---|
| Pre | Post | ||||
| Pain intensity | ULNTT 1, a- median nerve | 7 (8-5.75) | 2 (2-1) | 4.81 | 0.001 |
| ULNTT 1, b- median nerve | 7 (8-6) | 2 (2-1) | 4.81 | 0.001 | |
| ULNTT 2- radial nerve | 7 (8-6) | 1.5 (2-1) | 4.83 | 0.001 | |
| ULNTT 3- ulnar nerve | 6 (7-4.5) | 2 (2-1) | 4.81 | 0.001 | |
Table 2: Comparison between pre- and post-treatment median values of pain intensity of study group.
| Pre | Post | MD | % of change | t- value | p-value | |
|---|---|---|---|---|---|---|
| X̅ ± SD | X̅ ± SD | |||||
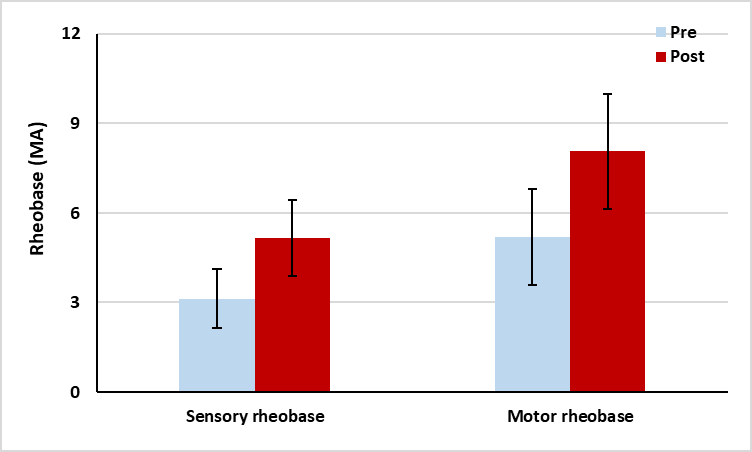
| Sensory rheobase (MA) | 3.13 ± 1 | 5.16 ± 1.26 | -2.03 | 64.86 | -9.6 | 0.001 |
| Motor rheobase (MA) | 5.2 ± 1.6 | 8.06 ± 1.92 | -2.86 | 55 | -8.84 | 0.001 |
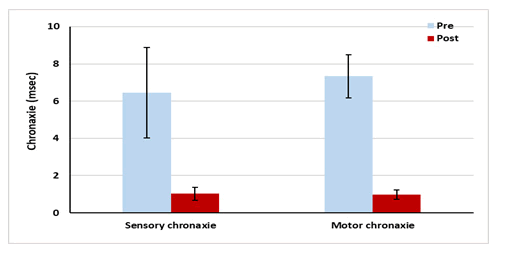
| Sensory chronaxie | 6.46 ± 2.43 | 1.03 ± 0.35 | 5.43 | 84.06 | 12.43 | 0.001 |
| Motor chronaxie | 7.33 ± 1.15 | 0.98 ± 0.26 | 6.35 | 86.63 | 29.44 | 0.001 |
| SD: Standard Deviation; MD: Mean Difference; p value: probability value; X: mean. | ||||||
Table 3: Comparison between pre and post treatment mean values ± SD of sensory and motor rheobase and chronaxie of the study group.
Effect of treatment on strength duration curve parameters
There was a significant increase in the post-treatment sensory (64.86%) and motor (55%) rheo base values and a significant decrease in sensory (84.06%) and motor (86.63%) chronaxie values as compared with that of pre-treatment values (p<0.001) [Figures 8 and 9].
Discussion
The current study was conducted to assess the effect of SURE on brachial plexus mechano sensitivity in subjects with SDR. According to our results, there was a significant increase in post-treatment sensory and motor rheo base values and a significant decrease in post-treatment sensory and motor chronaxie values after performing SURE compared with the same values pre-treatment respectively (p<0.001). Many studies carried out earlier, have yielded clinical evidence of using electro diagnostic measures such as SDC properties, Rheobase and chronaxie values, that are considered valid measure for excitability in diagnosing peripheral nerve mechanosensitivity which show variations in various pathological conditions. [19,20] The alteration in extracellular electrolyte concentration that is usually noticed in musculoskeletal pain conditions and hence membrane depolarization, and increased axonal excitability, might explain such changes in rheobase and chronaxie values in different neuro musculoskeletal conditions. Within our knowledge, even an asymptomatic scapular deviation, might show the same excitability response observed in musculoskeletal pain conditions as it applies an overdue load on muscles and nerves that would eventually result in muscle spasm and increased firing or excitability rate. [21] Sensory rheobase can be used to determine the pain threshold in both normal healthy subjects and persons with neuromuscular pain. It has been reported that sensory rheobase decreases in subjects suffering from trapezius spasm compared to normal healthy ones which is supporting our results not only in increasing sensory rheobase level post-treatment, but also in decreasing pain intensity and improving pain quality as a response to neuro dynamic test post SURE intervention. Persson et al. agreed with our results concerning increasing sensory rheobase values post-treatment, as he assessed fatigued muscles by Pressure Pain Threshold (PPT) and Electromyography (EMG). On static endurance test, consisting of sub-maximal unilateral activation of right trapezius and deltoid muscles and measuring PPT bilaterally on both muscles using an electronic pressure algometer and electromyography, Persson et al. found bilateral increase in PPT that could be explained by central pain regulating mechanism which is necessary for both sensory function in normal healthy persons as well as subjects with musculoskeletal pain. [22]
In their study on trapezius myalgia, Rosendal et al. have concluded that in cases of myo fascial trapezius pain, a significantly higher levels of interstitial protons, calcitonin gene related peptide, bradykin, substance P, tumor necrosis factor alpha, interleukin beta, serotonin, and norepinephrine are noted. [23] These pathologically induced biochemical changes results in inactivation of voltage-dependant Na+ channels which easily and readily explains the alterations in impulse transmission during muscle contraction, and thus the changes in parameters of the SDC. The muscle spasm induced pain is primarily due to ischemia leading to drop in extracellular pH and hence release of pain-producing substances such as bradykin, adenosine triphosphate and H+. These biochemical changes impose an increased excitability state, so that when the trapezius muscle is electrically stimulated, the already existing Na+ ions result in earlier contraction as compared to normal muscles. [24] As rheo base values depends on electrical impedance and excitability state of the muscle, while chronaxie values depends on the time relations of excitation process, this explanation properly supports the findings of the current study that abnormally overstretched trapezius muscle shows lower motor rheo base and higher motor chronaxie values. [25]
Some studies discussed differences existing between electrophysiological response of sensory and motor fibers and some research has been done to assess electrophysiological changes at a painful musculoskeletal site. [20] Salian et al. compared the normative motor and sensory SDC parameter values of trapezius muscle in asymptomatic persons suffering from chronic bilateral trapezius spasm and lower motor and sensory values of rheo base were reported, whereas, both motor and sensory chronaxie values, rise time and pulse ratio were increased in subjects with trapezius spasm as compared to normal healthy subjects. Thus, such comparison of SDC parameters between apparently healthy subjects and those with trapezius spasm, determines that there were changes in the SDC properties in presence of pain and muscle spasm and concludes that these properties can be used as an objective tool for the measurement of pain and prognosis.
In consistency with the findings of the current study, Lee et al. have assessed the influence of the depressed scapular alignment on muscle tenderness using (PPT) and delayed muscle activation using surface electromyography to measure Muscle Latency Time (MLT). They compared PPT and MLT in 10 patients with scapular depressed position to 10 normal subjects, and concluded that, patients with scapular depressed position showed significantly lower PPT scores than normal in both upper and middle trapezius. Also, the patient group showed a significantly increased MLT of the upper trapezius during shrug movement. [10] This recorded delayed muscle contraction timing could be attributed to the upper trapezius muscle adaptation to other recruitment models to maintain scapular stability during scapular movement, e.g., shrug motion. Other explanation of such recruitment delay, is the eventually occurring limited cross-bridge structures to produce force in response to subjecting the muscle to a prolonged or constant elongation position, considering that, the rate of cross-linking formation at the sarcomere influences to great extent the rate of muscle contraction. [10]
When the muscle undergoes prolonged stretch or prolonged elongated position, it exhibits an over-stretch weakness, which is considered a mild form of strain in the muscle tissue that hinder the performance of eccentric contraction against load. The current findings are consistent with a previous report by Proske et al. These conditions are potential for the development of painful muscle strain if not treated quickly when a change in muscle length occurs. Therefore, in order to improve muscle performance in such cases, the corrective strategy should put the aim to shorten the lengthened muscles to a more normal length in focus. [26] The alignment exercise of the scapula improves muscle performance by maintain nearly normal length of the stretched muscles, and thus regaining a more normal scapular and shoulder joint movement pattern. [27] According to Azeve et al. a prolonged upper trapezius muscle condition could negatively affect simple and regular movements critically involved activities of daily living, resulting in lower PPT values in these subjects. Nociceptors’ sensitization, especially muscle nociceptors, could explain lower PPT values observed. As muscle nociceptors become sensitized, they become able to demonstrate lower mechanical threshold and respond to weak muscle tissue deformation that would not otherwise evoke response in such nociceptors. [13] Further explanation of the impact of musculoskeletal pain on rheo base and chronaxie values, could strongly be attributed to an influx of nerve impulses into the spinal cord from muscle nociceptors that enhances the excitability of posterior horn neurons more than one from cutaneous nociceptors. Muscle pain chronification starts classically with increased excitability of spinal neurons and propagation of excitation throughout the CNS. [24]
According to our results, there was a significant decrease in pain intensity of median, radial and ulnar nerves post-treatment compared with that of pre-treatment as well as a significant improvement in pain quality of median, radial and ulnar nerves post-treatment compared with that of pre-treatment. Pain quality shifted from burning, numbness, pricking, tingling and pain to stretching in most of cases post-treatment. Physical loads on the nerve trunk may change as a result of posture and limb motions, resulting in changes in the structural and biomechanical features of the nerve as the “force or load acting on a specific area of tissue” is defined as physical stress.”
Mueller and Maluf in their physical stress theory stated that “the adaptive response of the biological tissue will depend on the physical stress applied on that particular tissue”. Neural tissues can adapt to applied mechanical stresses according to the magnitude of force applied, which configure three distinct concepts in this regard; (1) Excessive neural tissue elongation, if the force applied is more than necessary, (2) No adaptation if force is just adequate, and (3) Diminuted ability to tolerate stress if the force applied is less than tissue resistance. These responses are referred to neural tissue plasticity that conforms and adapts to the applied functional demands. A gliding mechanism of nerve tissue occurs during normal joint movement as a form of adaptation, allowing movement with minimum tension or without imposing any tension on individual nerve fibers. In addition, when compared to the nerve root, the nerve trunk extends to around 6%-8% of its original length, providing it more resistance to mechanical pressure. Micro vascular circulation is impaired by increased and repetitive elongation and compression, which can lead to a variety of complications as perineural oedema, neural connective tissue fibrosis, and degeneration of nerve fibers. Alterations in microcirculation and tissue pressures around the nervous systems are explicitly identified before the structural changes starts to take place. [28] As 86% of patients had a complaint of tingling through burning during the application of neurodynamic tension tests of median nerve (ULTT 1a,b), that indicates irritation being induced in upper limbs’ peripheral nerves attributed to scapular downward rotation alignment that creates tension on the C5 and C6 vertebral nerves, lateral cord of the brachial plexus, and the median nerve as it courses through the forearm. [29] The scapular downward rotation alignment can impose prolonged and repetitive stress and pressure on the brachial plexus, sensitize nervous tissue, and given time, may commence neck-arm pain symptoms. [1]
The sensitivity of the nervous tissue to this improper alignment was assessed through neuro dynamic tests for the peripheral nerve trunks of the arm. The findings of the current study were consistent with a previous study that identified high pain during the conduction of the neuro dynamic tests in asymptomatic SDR subjects compared with normal scapular alignment subjects. This confirms the impact of SDR on exaggerating the mechanical sensitivity of the nervous tissues of the neck and arms. Azevedo et al., have reported increased upper trapezius pain sensitivityin young healthy adults complaining of scapular depression [13,16] agree with our results, that the most common sensory response reported by subjects with and without depressed scapular alignment with the application of the ULTT was “stretching”. The term “stretching” does not reflect direct neurological involvement, so the stretching sensory response felt by the patient during the application of the ULTT might not be solelyof neural tissue origin. Explained that the terms “tingling” and “burning”, were the most commonly encountered neurogenic component of the symptom responses reported by the SDR alignment patients. [19,17] This finding is consistent with the results reported in the current study in term of the greater neural tissue sensitization which provide solid evidence that asymptomatic individuals showed elevated levels of neural mechano sensitivity and have reported a variety of sensory responses at the end range of neurodynamic tests.
Many previous studies have discussed the effects of modifying scapular alignment by passively bringing the scapula to normal alignment and maintaining the correct position with the assistance of the examiner. In conclusion, they suggested that maintaining the upward rotational position of the scapula through alignment exercise is a beneficial exercise method as it reduces the pressure in the cost clavicular space and alleviates the symptoms of thoracic outlet syndrome. [30-35] Despite the evidence of immediate benefits obtained through such passive interventions in terms of decreasing scapular depression and reducing neck-shoulder symptoms and dysfunction, long–term effects are still lacking. [7,8] Exercise interventions have shown being necessary to obtain long-term effects, especially to restore optimal muscle status in the form of optimal strength and length for execution of normalfunctional motion in the upper–extremity. Stretching and strengthening exercise programs are often utilized as essential component of the conservative treatment of shoulder pain as they are capable of reducing symptoms and correct abnormal motion and muscle action. So, to ensure effective approach of correcting anatomic length adaptation is to facilitate lengthened muscles to assume a shortened position and simultaneously stretch the shortened muscles. Scapular Upward Rotation Exercise (SURE), opposes the effects of SDR on peri-scapular muscles, through augmenting serratus anterior and upper trapezius strength (scapular upward rotators) and stretches levator scapulae (scapular downward rotators), which restores optimal functional scapular position in terms of muscle length and strength in patients with SDR [7,8,11] demonstrated that the 6 weeks SURE strengthening exercise program for SDR correction have yielded an increase in serratus anterior and upper trapezius strength grade, as defined by Kendall. The SURE was thought to enhance muscle strength in the serrat us anterior and upper trapezius muscles, as well as extend the levator scapula, by modifying the resting muscle length and reducing the number of sarcomeres.
Passive correction of downwardly rotated scapula, is thought to reduce symptoms of neck pain, and enhance proprioception, which in turn increases active neck rotation, in neck-pain patients with bilateral scapular downward rotation syndrome, [7,8] which augments the findings of the current study.
In participants with SDR syndrome, Ha et al. discussed the effects of SURE on scapular and clavicular alignment, as well as the strength of scapular upward rotators and found significant variations in scapular and clavicular alignment, as well as the strength of scapular upward rotators, between pre and post intervention recordings and thus giving evidence for the effectiveness of SURE in subjects with SDR. [7,8]
Watson et al. found that the improvement achieved by manual correction of the scapula directs the diagnosis of thoracic outlet syndrome, as it mitigates the irritation induced on peripheral nerves in such syndrome, in addition to that, it is a very useful clinical indication of the effectiveness of rehabilitation strategy conducted focusing on strengthening the scapular stabilizers, and whether the change in scapular posture in both rest and motion states would be successful. Maintaining the alignment exercise for one minute, could relieve the traction force acting on the brachial plexus. Therefore, when a slight increase in the PTT on the median nerve is recorded in patients after the application of scapular alignment exercises, can be attributed to the decrease in mechanical sensitivity of peripheral nerve roots, and nerve trunks of the brachial plexus owing to releasing the traction and elongation forces acting on them.
For enhancing the activation of the scapular upward rotators, the shrug exercise with a 30° shoulder abduction is recommended. Although it has been demonstrated that the shrug with 30° abduction is preferable than the traditional shrug, additional shrug exercises may even have greater benefits for training the upper trapezius and scapular upward rotators. Previous researchers proved that during arm abduction in the frontal plane, upper trapezius muscle activity was higher compared to the sagittal plane.
Conclusion
The authors of the current study conclude that (SURE) restore the lost peri-scapular muscular balance and hence reducing mechano sensitivity of the brachial plexus in scapular depressed alignment subjects. This study recommends that SURE could help scapular depression syndrome patients as a therapeutic exercise method in clinical field, and that regaining normal scapular alignment is an integral part of the physiotherapy program of cervical problems as well as upper limp complaints with cervical origin, and they should be incorporated within physiotherapy clinical plan.
References
- Van Dillen LR, McDonnell MK, Susco TM, Sahrmann SA. The immediate effect of passive scapular elevation on symptoms with active neck rotation in patients with neck pain. Clin J Pain. 2007;23:641-47. [Crossref], [Google Scholar], [Indexed]
- Kim TH, Lim JY. The effects of wall slide and sling slide exercises on scapular alignment and pain in subjects with scapular downward rotation. J Phys Ther Sci. 2016;28:2666-2669. [Crossref], [Google Scholar], [Indexed]
- Caldwell C, Sahrmann S, Van Dillen L. Use of a movement system impairment diagnosis for physical therapy in the management of a patient with shoulder pain. J Orthop Sports Phys Ther. 2007;37:551-563. [Crossref], [Google Scholar], [Indexed]
- Rich RL, Struminger AH, Tucker WS, Munkasy BA, Joyner AB, Buckley TA. Scapular upward-rotation deficits after acute fatigue in tennis players. J Athl Train. 2016;51:474-479. [Crossref], [Google Scholar], [Indexed]
- Yayama T, Kobayashi S, Nakanishi Y, Uchida K, Kokubo Y, Miyazaki T, et al. Effects of graded mechanical compression of rabbit sciatic nerve on nerve blood flow and electrophysiological properties. J Clin Neurosci. 2010;17:501-5. [Crossref], [Google Scholar], [Indexed]
- Nee RJ, Vicenzino B, Jull GA, Cleland JA, Coppieters MW. Baseline characteristics of patients with nerve-related neck and arm pain predict the likely response to neural tissue management. J Orthop Sports Phys Ther. 2013;43:379391. [Crossref], [Google Scholar], [Indexed]
- Ha SM, Kwon OY, Yi CH, Jeon HS, Lee WH. Effects of passive correction of scapular position on pain, proprioception, and range of motion in neck-pain patients with bilateral scapular downward-rotation syndrome. Man Ther. 2011;16:585e589. [Crossref], [Google Scholar], [Indexed]
- Ha SM, Kwon OY, Yi CH, Cynn HS, Weon JH, Kim TH. Effects of scapular upward rotation exercises on alignment of scapula and clavicle and strength of scapular upward rotators in subjects with scapular downward rotation syndrome. Journal of Electromyography and Kinesiology 2016; 26: 130-136. [Crossref], [Google Scholar], [Indexed]
- Kang MH, Choi JY, Oh JS. Effects of crossed brassiere straps on pain, range of motion, and electromyographic activity of scapular upward rotators in women with scapular downward rotation syndrome. Randomized Controlled Trial PM R. 2015;7:1261-1268. [Crossref], [Google Scholar], [Indexed]
- Lee KT, Chuang CC, Lai CH, Ye JJ, Wu CL. Study of the trapezius muscle region pressure pain threshold and latency time in young people with and without depressed scapula. Man Ther. 2015;20:124-9. [Crossref], [Google Scholar], [Indexed]
- Nee RJ, Jull GA, Vicenzino B, Coppieters MW. The validity of upper-limb neurodynamic tests for detecting peripheral neuropathic pain. J Orthop Sports Phys Ther. 2012; 42:413-424. [Crossref], [Google Scholar], [Indexed]
- Andrade GT, Azevedo DC, De Assis LI, Neto RSG, Sadala Do PV, Gonçalves RTF, et al. Influence of scapular position on cervical rotation range of motion. J Orthop Sports Phys Ther. 2008;38:668-673. [Crossref], [Google Scholar], [Indexed]
- Azevedo DC, De Lima PT, De Souza AF, Mc Donnell MK. Influence of scapular position on the pressure pain threshold of the upper trapezius muscle region. Eur J Pain. 2008;12:226-32. [Crossref], [Google Scholar], [Indexed]
- DiVetaJ, Walker ML, Skibinski B. Relationship between performance of selected scapular muscles and scapular abduction in standing subjects. Phys Ther. 1990;70:470-476. [Crossref], [Google Scholar], [Indexed]
- Greenfield B, Catlin PA, Coats PW, Green E, Mc Donald JJ, North C. Posture in patients with shoulder overuse injuries and healthy individuals. J Orthop Sports Phys Ther. 1995;21:287-295. [Crossref], [Google Scholar], [Indexed]
- Lohkamp M, Small K. Normal response to upper limb neurodynamic Test 1 and 2A. Man Ther. 2011; 16:125-30. [Crossref], [Google Scholar], [Indexed]
- Martínez MD, Cubas CL, Girbés EL. Ulnar nerve neurodynamic test: study of the normal sensory response in asymptomatic individuals. J Orthop Sports Phys Ther. 2014;44:450-6. [Crossref], [Google Scholar], [Indexed]
- Oliver GS, Rushton A. A study to explore the reliability and precision of intra and inter-rater measures of ULNT1 on an asymptomatic population. Man Ther. 2011;16:203-6. [Crossref], [Google Scholar], [Indexed]
- Martínez MP, Lluch E, Gallezo IT, Pecos MD, Plaza MG, Nuñez NS, et al. The influence of a depressed scapular alignment on upper limb neural tissue mechanosensitivity and local pressure pain sensitivity. Musculoskelet Sci Pract. 2017;pp:60-65. [Crossref], [Google Scholar], [Indexed]
- Genç G, Bek S, Kasikci T, Ulas UH, Demirkaya S, Odabasi Z. Strength duration time constant in peripheral nerve: No abnormality in multiple sclerosis. Mult Scler Int. 2012;390-157. [Crossref], [Google Scholar], [Indexed]
- Mogyoros I, Kiernan MC, Burke D, Bostock H. Strength-duration properties of sensory and motor axons in amyotrophic lateral sclerosis. 1998;121:851-859. [Crossref], [Google Scholar], [Indexed]
- Persson AL, Hansson GA, Kalliomäki J, Moritz U, Sjölund BH. Pressure pain thresholds and electromyographically defined muscular fatigue induced by a muscular endurance test in normal women. Clin J Pain. 2000;16:155-163. [Crossref], [Google Scholar], [Indexed]
- Rosendal L, Kristiansen J, Gerdle B, Søgaard K, Peolsson M, Kjaer M. Increased levels of interstitial potassium but normal levels of muscle IL 6 and LDH in patients with trapezius myalgia. Pain. 2005; 119:201 9. [Crossref], [Google Scholar], [Indexed]
- Mense S. Muscle pain mechanisms and clinical significance. Dtsch Arztebl Int. 2008;105:214. [Crossref], [Google Scholar], [Indexed]
- Buchanan DN, Garven HS. The chronaxie in tetany: The effect on the chronaxie of thyreoparathyreoidectomy, the administration of guanidin and of di-methyl guanidin. J Physiol. 1926; 62:115. [Crossref], [Google Scholar], [Indexed]
- Proske U, and Morgan DL. Muscle damage from eccentric exercise: Mechanism, mechanical signs, adaptation and clinical applications. J Physiol. 2001;537:333-345. [Crossref], [Google Scholar], [Indexed].
- Wegner, S., Jull, G., O'Leary, S., & Johnston, V. The effect of a scapular postural correction strategy on trapezius activity in patients with neck pain. Manual therapy 2010, 15(6): 562-566. [Crossref], [Google Scholar], [Indexed]
- Topp KS, Boyd BS. Structure and biomechanics of peripheral nerves: Nerve responses to physical stresses and implications for physical therapist practice. Phys Ther. 2006;86:92-109. [Crossref], [Google Scholar], [Indexed]
- Byl C, Puttlitz C, Byl N, Lotz J, Topp K. Strain in the median and ulnar nerves during upper-extremity positioning. J Hand Surg. 2002;27:1032-1040. [Crossref], [Google Scholar], [Indexed],
- Kitamura T, Takagi K, Yamaga M, Morisawa K. Brachial plexus stretching injuries: microcirculation of the brachial plexus. J Shoulder Elbow Surg. 1995;4:118-123. [Crossref], [Google Scholar], [Indexed]
- Watson LA, Pizzari T, Balster S. Thoracic outlet syndrome part 2: Conservative management of thoracic outlet. Man Ther. 2010;15:305-314. [Crossref], [Google Scholar], [Indexed]
- Pizzari T, Wickham J, Balster S, Ganderton C, Watson L. Modifying a shrug exercise can facilitate the upward rotator muscles of the scapula. Clin Biomech. 2014; 29:201-5. [Crossref], [Google Scholar], [Indexed]
- Dilley A, Bove GM. Disruption of axoplasmic transport induces mechanical sensitivity in intact rat C-fibre nociceptor axons. J Physiol 2008;586(2):593- 604. [Crossref], [Google Scholar], [Indexed]
- Lewis JS, Wright C, Green A. Subacromial impingement syndrome: The effect of changing posture on shoulder range of movement. J Orthop Sports Phys Ther. 2005;35:72-87. [Crossref], [Google Scholar], [Indexed]
- Moses A, Carman J. Anatomy of the cervical spine: Implications for the upper limb tension test. Aust J Physiother. 1996; 42:31-5. [Crossref], [Google Scholar], [Indexed]




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.