Evaluation of Phytochemical Screening and Anti Inflammatory Activity of Leaves and Stem of Mikania scandens (L.) Wild
- *Corresponding Author:
- Dr. Soma Banerjee
Department of Biotechnology, Heritage Institute of Technology, Chowbaga Road, Anandapur, Kolkata - 700 107, West Bengal, India.
E-mail: soma.banerjee@heritageit. edu
Abstract
Background: The greatest disadvantage in the presently available potent synthetic anti‑inflammatory drugs lies in their toxicity and reappearance of symptoms after discontinuation. Hence, people are returning to the natural products with the hope of safety and security. Several species of Mikania have been reported to have anti‑inflammatory properties. Aim: The present study aims to assess the anti‑inflammatory activity of the ethanolic extract of the leaves and stem of Mikania scandens in vivo and in vitro. Materials and Methods: The in vitro bioassay consisted of assaying the effect of the extracts against denaturation of protein (egg albumin) and measuring the absorbance. In vivo anti‑inflammatory activity was checked by measuring the percentage inhibition of carrageenan‑induced rat paw edema after oral administration of the extracts to male Wistar rats. Results: The plant extracts revealed the presence of tannins, alkaloids, steroids and flavonoids in both the leaf and stem extracts. The in vitro study of leaf extracts of M. scandens demonstrated that at 16000 μg/ml concentration a better anti‑inflammatory activity was exhibited which is more than the stem extracts. Similarly in the in vivo study, carrageenan induced inflammation was significantly antagonized by M. scandens leaf extract, with inhibition of 50% at 1000 mg/kg. Conclusion: The ethanolic extract of both leaf and stem of M. scandens showed potent anti‑inflammatory activity. In comparison the leaf extract found to be more potent in both the conditions in vivo and in vitro, comparing with the standard drug diclofenac sodium and traditional control rumalaya perhaps due to the presence of phytochemicals like alkaloids and flavonoids in the plant.
Keywords
Anti-inflammatory, Mikania scandens (L.) wild, Phytochemicals, Protein denaturation assay, Rat paw edema
Introduction
Inflammatory diseases including different types of rheumatic diseases are a major cause of morbidity of the working force throughout world. This has been called the “King of Human Miseries.”[1] The attention of pharmacologists throughout the world has been focused on finding out safer and potent anti-inflammatory drug. The most commonly used drugs for management of inflammatory conditions are non-steroidal anti-inflammatory drugs (NSAIDs).[2,3] The greatest disadvantage in the presently available potent synthetic anti-inflammatory drugs lies in their toxicity and reappearance of symptoms after discontinuation.[4,5] The natural products today symbolize safer in contrast to the synthetic drugs that are regarded as unsafe to humans and environment. Hence, people are returning to the natural products with the hope of safety and security.
Mikania scandens belongs to the family Asteraceae and is a fast growing perennial creeper. M. scandens is natively found in the tropical and sub-tropical zones of North, Central and South America. This genus is also found in the tropics of Africa and Asia [6] and is widely known as Guaco. It comprises about 450 identified species.[7] There has been several reports on the use of other species of Mikania as a folkloric medicine in different therapeutic applications by different groups.[8,9]
Different extracts of M. scandens have been shown to have significant medicinal properties including antimicrobial, eupeptic and antispasmodic effects.[10-12] Presence of anti-inflammatory property of several species of Mikania has been observed.[11,13,14]
The present study aims to assess the anti-inflammatory activity of the ethanolic extract of both the leaves and stem of M. scandens in vivo and in vitro and also of diclofenac sodium as a standard NSAID on the basis of previous reports of its use for the same purpose.[15,16] The presence of some phytochemical components was also determined.
Materials and Methods
Plant material collection and extraction
Whole plant was collected from East Kolkata Wetlands area in Kolkata. Voucher specimen no: 20007 CUH was deposited in the herbarium of Department of Botany of University of Calcutta, West Bengal, India. The leaves and stem were separated, washed and dried at 50°C ± 5°C in a hot air oven until all water was evaporated. They were separately weighed and macerated in 70% aqueous ethanol and kept for 72 h submerged in the same solvent at 4°C. Then they were filtered and the extract was stored at 4°C.
Drugs and chemicals
Standard drug used was diclofenac sodium (Voveran 50, Novartis India Ltd., Mumbai, India). Traditional drug used was Rumalaya Forte tablets which are a potent and safe phytopharmaceutical formulation that relieves joint and bone aches associated with various orthopedic ailments. As per literature, it is a combination of natural ingredients including Boswellia’s (Shallaki) gum resin extract and Indian Bdellium (Guggul) both act as anti-inflammatory agent. All chemicals used in the study were of analytical grade.
Experimental animals
Male Wistar rats (Rattus norvegicus) with average weight of 120-130 g were obtained from R.G. Kar Medical College and Hospital, Kolkata. The animals were maintained there in animal house at controlled temperature, humidity, light-dark cycle and were allowed free access to food and water for 15 days according to Committee for the Purpose of Control and Supervision of Experiments on Animals guidelines. A total of 48 male rats were randomized divided into 8 groups of 6 animals each. The extracts were administered orally into the rats using rat feeding cannula throughout the experimental period. Group 1 (neutral control): Received distilled water orally (10 ml/kg); Groups 2 (positive control): Received diclofenac sodium (12.5 mg/kg) orally;[17] Groups 3 (traditional standard): Received Rumalaya Forte (12.5 mg/kg) orally; Group 4: Received Gum acacia (0.5 mg/100 g) as vehicle. Groups 5 and 6 were treated orally with ethanolic extract of leaf (doses 500 mg/kg and 1000 mg/kg body weight) respectively; Group 7 and 8 were treated orally with ethanolic extract of stem (doses 500 mg/kg and 1000 mg/kg body weight) respectively. Ethical clearance was obtained from R. G. Kar Medical College Institutional Animal Ethical Committee.
Preliminary phytochemical analysis
The extracts were subjected to preliminary phytochemical screening for the detection of major chemical groups. Plant extract was taken and tested for tannin, alkaloids and steroids following standard protocol [18] similarly for flavonoids plant extract were tested with 5% sodium hydroxide solution followed by 2 ml of 10% hydrochloric acid following the standard protocol.[18]
Anti-inflammatory activity
Anti-inflammatory bioassay in vitro
According to previously reported protocol,[19] The reaction mixture consisted of 0.2 ml of egg albumin (from fresh hen’s egg), 2.8 ml of phosphate buffered saline (pH 6.4) and 2 ml of varying concentrations of the test extract, by which the concentrations (μg/ml) became 400, 800, 2000, 4000, 8000, 16,000. This range was chosen because concentrations below 400 μg/ml gave very insignificant inhibition and above 16,000 μg/ml the value became too high. Similar volume of double-distilled water served as control. Then the mixtures were incubated at 37°C ± 2°C in a biological oxygen demand incubator for 15 min and then heated at 70°C for 5 min. After cooling, their absorbance was measured at 660 nm (Systronix Spectrophotometer 150) by using vehicle as blank. Diclofenac sodium and rumalaya forte at the final concentrations (μg/ml) of 50, 100, 250, 500, 1000, 2000 were used as reference and traditional/herbal drugs respectively and treated similarly for determination of absorbance. Test extracts were chosen such that, they remained the nearest possible to the standard therapeutic mode. The percentage inhibition of protein denaturation was calculated by using the following formula:
% inhibition = 100 × ([Vt/Vc] –1).
Where, Vt = absorbance of test sample, Vc = absorbance of control.
Carrageenan-induced rat paw edema
Carrageenan was freshly prepared in normal saline (1%) and after 1 h of extract administration; 0.1 ml of 1% carrageenan suspension was injected subcutaneously into plantar surface of the left hind paw of experimental animals under mild ether anesthesia. The volume of the paw up to ankle joint was measured 1 h prior to the injection (V0) and hourly up to 3 h (Vt) after the injection of carrageenan, using a plethysmometer.[20] The percentage inhibition of edema was calculated using these paw volumes, with respect to their controls.
% edema inhibition = (Vt − V0) control − (Vt − V0) treated/ (Vt − V0) control × 100.
Statistical analysis
All data obtained were expressed as mean (standard deviation). The results were analyzed for statistical significance Student’s t-test. using InStat version 3.0 (GraphPad Software Inc., La Jolla, CA, USA). P < 0.01 was considered as statistically significant.
Results
Preliminary phytochemical analysis
The extracts of M. scandens showed the presence of following phytochemicals, i.e., alkaloids, flavonoids, tannins and steroids [Table 1].
| Plant part | Alkaloids | Tannins | Steroids | Flavonoids |
|---|---|---|---|---|
| Leaf | +ve | +ve | +ve | +ve |
| Stem | +ve | +ve | +ve | +ve |
+ve: Presence, -ve: Absence
Table 1: Phytochemical properties of Mikania scandens leaves and stem extract
Anti-inflammatory bioassay in vitro
Leaf extracts of M. scandens at the dose of 16000 μg/ml exhibited an anti-inflammatory activity that became significant (P < 0.01) with a 65% more inhibitory effect than the stem extract as presented in Table 2.
| Concentration (µg/ml) | % inhibition (leaf) | % inhibition (stem) |
|---|---|---|
| Control | - | - |
| 400 | 5.88 (0.04)* | 1.96 (0.01)* |
| 800 | 7.84 (0.08)* | 3.92 (0.03)* |
| 2000 | 17.64 (0.13)* | 7.84 (0.05)* |
| 4000 | 56.86 (0.38)* | 27.45 (0.22)* |
| 8000 | 154.9 (1.16)* | 66.66 (0.71)* |
| 16000 | 435.29 (3.49)* | 150.98 (1.28)* |
Each point represents the mean (SD), n=5. The data was analyzed by student’s t test. Asterisks indicated statistically significant values from control. *P<0.01. SD: Standard deviation
Table 2: Influence of extracts of Mikania scandens against protein denaturation
Percentage inhibition of standard drugs
The extract (1000 and 2000 μg/ml) induced significant (P < 0.01) anti-inflammatory effect. The anti-inflammatory effect of positive control (diclofenac) at concentration of 2000 μg/ml was 13.32% greater than that of the traditional standard (Rumalaya).
Half maximal inhibitory concentration (IC50) values extracts of M. scandens and standard drugs
IC50 values indicate that the standard drug (Rumalaya) and the positive control (Diclofenac sodium) is approximately 10 times more potent than the crude stem extract and 6 times more potent than crude leaf extracts respectively. Hence, with respect to the plant samples leaf extracts is showing to be 75.67% more potent than the stem extract.
All values have P < 0.01 as compared to control (rat not given any treatment).
Discussion
Denaturation of tissue proteins may be the cause behind the production of auto-antigens in certain arthritic diseases. So it may be said that tissue protein denaturation is a marker for inflammatory and arthritic diseases.[19] Agents that can prevent protein denaturation, therefore, would be possible candidate for anti-inflammatory drug development. With this idea in mind, the in vitro test was done as a preliminary screen to check presence of anti-inflammatory property before doing the in vivo test. In the present study, the protein denaturation bioassay was selected for in vitro assessment of anti-inflammatory property of ethanolic extract of M. scandens aerial parts with a wide range of dose concentrations.
The present findings exhibited a concentration dependent inhibition of protein (albumin) denaturation by both the test extracts throughout the concentration range of 400-16000 μg/ml [Table 2]. Diclofenac sodium (at the concentration range of 50-2000 μg/ml) was used as the standard drug, which also exhibited concentration dependent inhibition of protein denaturation [Table 3]. This was chosen as a standard NSAID on the basis of previous reports of its use for the same purpose.[15,16] The increased absorbance in both the extracts and the standard drug with respect to control indicates the protein stabilizing activity (denaturation is inhibited) with increased dose. Table 4 explains that the IC50 values indicate that the positive control (Diclofenac sodium) and the standard drug (Rumalaya) is approximately 6 times more potent than the crude leaf extract and 10 times more potent than crude stem extracts respectively. So, with respect to the plant samples leaf extracts is showing to be 75.67% more potent than the stem extract. Hence it is obvious, that if these crude extracts are purified, the pharmacological activity will increase significantly and might even match those of the standard drug.
| Concentration | % inhibition | % inhibition | |
|---|---|---|---|
| (µg/ml) | (diclofenac) | (rumalaya) | |
| Control | - | - | |
| 50 | 11.9 (0.20)* | 9.28 (0.12)* | |
| 100 | 14.28 (0.22)* | 11.83 (0.18)* | |
| 250 | 23.8 (0.31)* | 19.19 (0.24)* | |
| 500 | 42.85 (0.38)* | 36.78 (0.31)* | |
| 1000 | 185.57 (1.19)* | 158.99 | (1.2)* |
| 2000 | 604.76 (4.51)* | 533.67 (4.81)* | |
Each point represents the mean (SD), n=5. The data was analyzed by Student’s t test. Asterisks indicated statistically significant values from control. *P<0.01. SD: Standard deviation
Table 3: Influence of standard drugs against protein denaturation
| Treatments | IC50 values (µg/ml) | |
|---|---|---|
| Leaf extract | 3700 | (25.34) |
| Stem extract | 6500 | (62.56) |
| Diclofenac sodium | 620 | (3.11) |
| Rumalaya forte | 685 (3.7) | |
IC50: Half maximal inhibitory concentration
Table 4: IC50 values extracts of Mikania scandens and standard drugs against protein denaturation
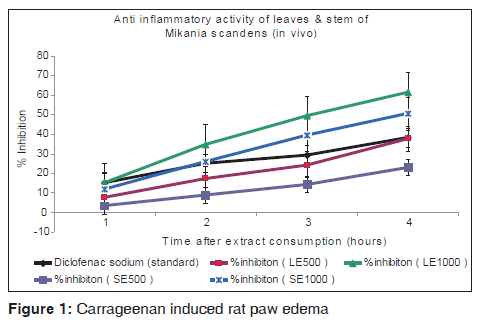
For assessment of anti-inflammatory effect of natural products as well as standard drugs carrageenan-induced paw edema is a well-established animal model. Edema formation is due to the effect of carrageenan in paw which is a biphasic event. The initial phase (1 h or 1.5 h) is mainly a non-phagocytic edema and a second phase (2-5) h is followed with increased edema formation that remains up to 5 h.[21,22] The initial phase is triggered due to the action of several mediators such as histamine, serotonin and bradykinin on vascular permeability.[23] The late phase or second phase edema is the result of overproduction of another mediator prostaglandins.[24] Taking into account the findings from the in vitro system, two doses were tested in vivo. It was observed [Figure 1] that, both the leaf and stem extracts reduced the carrageenan induced rat paw edema significantly when compared with the standard drug - diclofenac sodium. The result of pre-treatment of demonstrated that the extracts of both parts of the plant (500 and 1000 mg/kg i.p.) is effective in the early phase of inflammation, which is because of histamine and serotonin release. The anti-inflammatory effect of the extract remains significant up to 4th h of the experiment.
All the results were statistically significant (P < 0.01). The 1000 mg/kg dose of the leaf extracts shows 37.62% greater activity than the recommended dose of the standard drug (Diclofenac sodium). Similar observation of anti-inflammatory activity has been observed in different medicinal plants by different groups.[25]
During preliminary phytochemical screening of the crude extracts of both the leaves and stem parts, important therapeutic principles such as alkaloids, saponins, flavonoids, tannins etc., were detected. Therefore, the current findings can be attributed to these groups of chemical compounds. Further study is need on these plant extracts to find the exact mechanism of action for its pharmological properties over its anti-inflammatory effects.
Conclusion
Hence, it may be concluded from the present findings that the ethanolic extracts of both the leaves and stems of M. scandens have significant anti-inflammatory activity in both in vitro and in vivo systems. Further research on isolating the responsible components may be undertaken and they may be incorporated into existing anti-inflammatory herbal compositions to improve their efficacy.
Acknowledgment
The authors would like to thank to the authorities of the Heritage Institute of Technology, Kolkata and R.G. Kar Medical College and Hospital, Kolkata for providing the necessary facilities for the present study.
Source of Support: Heritage Institute of Technology, Kolkata and R.G. Kar Medical College and Hospital, Kolkata. Standard drug used was diclofenac sodium (Voveran 50, Novartis India Ltd., Mumbai, India). Traditional drug used was Rumalaya Forte (The Himalaya Drug Company, Bangalore, India).
Conflict of Interest: None declared.
References
- Chatterjee GK, Pal SP. Search for anti inflammatory agents from Indian medicinal plants a review. Indian Drugs 1984;21:413.
- Tripathi KD. Essentials of Medical Pharmacology. 6th ed. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd.; 2008.
- Bennett PN, Brown MJ. Clinical Pharmacology. 9th ed. New Delhi: Churchill Livingstone; 2005.
- Chawla AS, Handa SS, Sharma AK, Kaith BS. Plant anti-inflammatory agents. J Sci Ind Res 1987;46:214-23.
- Shen TY. Burger Award address. Toward more selective antiarthritic therapy. J Med Chem 1981;24:1-5.
- King RM, Robinson H. The Genera of the Eupatorieae (Asteraceae). St. Louis, MO: Missouri Botanical Garden; 1987.
- Holmes WC. A review preparatory toan infrageneric classification of Mikania (Tribe: Eupatorieae). In: Hind DJN, Jeffrey C, Pope GV (eds) Royal Botanical Gardens Kew, England, ; 1995. p. 239-54.
- Napimoga MH, Yatsuda R. Scientific evidence for Mikania laevigata and Mikania glomerata as a pharmacological tool. J Pharm Pharmacol 2010;62:809-20.
- Acree WE Jr. Toxicity and Drug Testing. 1st ed., Chap. 13. In Tech Publisher, New York; 2012. p. 297-320.
- Baral B, Bhattarai N, Vaidya GS. Pharmacological and antagonistic potentials of Mikania sp. Nepal J Sci Tech 2011;12:75-84.
- Hajra S, Mehta A, Pandey P, John J, Mehta P. Antibacterial property of crude ethanolic extract of Mikania micrantha. Asian J Exp Biol Sci 2010;1:166-8.
- Pérez-Amador MC, Ocotero VM, Balcazar RI, Jiménez FG. Phytochemical and pharmacological studies on Mikania micrantha HBK (Asteraceae). Int J Exp Bot 2010;79:77-80.
- Suyenaga ES, Reche E, Farias FM, Schapoval EE, Chaves CG, Henriques AT. Antiinflammatory investigation of some species of Mikania. Phytother Res 2002;16:519-23.
- Alves CF, Alves VB, de Assis IP, Clemente-Napimoga JT, Uber-Bucek E, Dal-Secco D, et al. Anti-inflammatory activity and possible mechanism of extract from Mikania laevigata in carrageenan-induced peritonitis. J Pharm Pharmacol 2009;61:1097-104.
- Sarma DS, Babu AV, Krishna KR. Antioxidant and anti inflammatory activities of Thespesia populnea linn. Int J Res Pharm Chem 2011;1:674-6.
- Kamble PS, Palatty PL, Kamble S. Comparative study of anti-inflammatory effects of rosiglitazone and pioglitazone with diclofenac sodium in carageenan induced rat hind paw oedema. Int J Bas Appl Med Sci 2012;2:1-7.
- Singh BT, Choudhary S, Vijayvergia R. In vitro anti-inflammatory, analgesic and acute toxicity studies of ethanol extracts of Andrographis paniculata Nees and Spilanthus acemella Murr. Der Pharmacia Sinica 2012;3:590-3.
- Al Nayeem A, Khatun A, Rahman MS, Rahman M. Evaluation of phytochemical and pharmacological properties of Mikania cordata (Asteraceae) leaves. J Pharm Phytother 2011;3:118-23.
- Dey P, Chatterjee P, Chandra S, Bhattacharya S. Comparative in vitro evaluation of anti-inflammatory effects of aerial parts and roots from Mikania scandens. J Adv Pharm Educ Res 2011;1:271-7.
- Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med 1962;111:544-7.
- Khan H, Khan MA, Muhammad N, Ashraf N, Gul F, Tariq SA. Anti inflammatory and antioxidant activity of Joshanda partially mediated through inhibition of lipoxygenase. Phytopharmacology 2012;3:19-28.
- Khan H, Saeed M, Mehmood MH, Rehman NU, Muhammad N, Ikram-Ul H, et al. Studies on tracheorelaxant and anti-inflammatory activities of rhizomes of Polygonatum verticillatum. BMC Complement Altern Med 2013;13:197.
- Maity TK, Mandal SC, Mukherjee PK, Saha K, Das J, Pal M, et al. Studies on anti inflammatory effect of Cassia tora leaf extract (fam. Leguminosae). Phytother Res 1998;12:221-3.
- Pérez-Guerrero C, Herrera MD, Ortiz R, Alvarez de Sotomayor M, Fernández MA. A pharmacological study of Cecropia obtusifolia Bertol aqueous extract. J Ethnopharmacol 2001;76:279-84.
- Muhammad N, Saeed M, Khan H. Antipyretic, analgesic and anti-inflammatory activity of Viola betonicifolia whole plant. BMC Complement Altern Med 2012;12:59.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.