Fabry's Disease: Case Series and Review of Literature
- *Corresponding Author:
- Dr. Riyaz Ahmad Bhat
Directorate of Health Services Kashmir, Srinagar , Jammu and Kashmir, India.
E-mail: bhatdrriaz@hotmail.com
Citation: Wani MM, Khan I, Bhat RA, Ahmad M. Fabry's disease: Case series and review of literature. Ann Med Health Sci Res 2016;6:193-7.
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Fabry’s disease is an X-linked lysosomal storage disorder caused by a deficiency of alpha-galactosidase A enzyme with the progressive accumulation of globotriaosylceramide in vascular endothelial cells leading to cardiovascular, renal, gastrointestinal, neuropathic, lenticular, and dermatological manifestations. It is a rare cause of end stage renal disease. It classically affects males whereas 10–15% of female heterozygote carriers are affected depending on localization. Both the FD and its association with ESRD is rare. With this background, this case series of five patient’s along with the review of literature is presented here.
Keywords
Alpha‑galactosidase A, End‑stage renal disease, Fabry’s disease, Kidney transplantation, Proteinuria
Introduction
Fabry’s disease (FD) is one of those rare disorders which are highly undiagnosed. The reported incidence of this disorder is 1/40,000 males.[1] FD being a rare cause of end‑stage renal disease (ESRD), accounts for 0.0167% of all causes of ESRD.[2] Manifestations of FD are often more severe in men due to the very low residual function of alpha‑galactosidase. Heterozygous females may be asymptomatic except for corneal opacities or depending on lyonization or random X‑inactivation, they may be as severely affected as homozygous males.[3]
We hereby report five patients with FD having ESRD. One of the patients received a human leukocyte antigen‑identical transplant from his brother whose pretransplant workup was negative but was not tested for FD. Posttransplant, the recipient, developed progressive worsening of serum creatinine and proteinuria and was biopsied. The biopsy showed features suggestive of acute T‑cell mediated rejection in allograft with diffuse vacuolization of visceral epithelial cells causing suspicion of a metabolic disease (e.g., FD). Subsequent enzymatic and pedigree analysis confirmed the diagnosis of FD in the recipient and one of his brothers with the possibility of their mother being an affected carrier.
Four years posttransplant, the patient continues to have progressively worsening serum creatinine. The other four patients were diagnosed on the basis of clinical suspicion and confirmed by low enzymatic levels of alpha‑galactosidase A. The pedigree analysis revealed that the male members of the family had low enzyme levels and are being planned for treatment with enzyme replacement therapy (ERT). Three patients are described below.
Case Reports
Case 1
The patient is a 45‑year‑old male who presented in 2009 with proteinuria, high serum creatinine and bilateral shrunken kidneys on ultrasonography (USG) and was diagnosed as ESRD. Etiology was presumed to be chronic glomerulonephritis – exact etiology was not known. The patient was put on continuous ambulatory peritoneal dialysis protocol before transplant and received a living‑related kidney transplant from his brother in 2009 with excellent immediate graft function.
The patient’s immunosuppression regimen comprised pulse steroid therapy followed by triple immunosuppression regime (prednisone, tacrolimus, and mycophenolate mofetil). No induction with anti‑thymocyte globulin/monoclonal antibodies was given. During his 1st year posttransplant, he developed polycythemia which was managed with phlebotomy. After 1½ year, his serum creatinine reached 2.0 mg/dl. He was noted to have significant proteinuria of 4 g/day. His general physical and systemic examination was normal. The patient underwent a renal transplant biopsy to ascertain the cause of worsening creatinine and proteinuria.
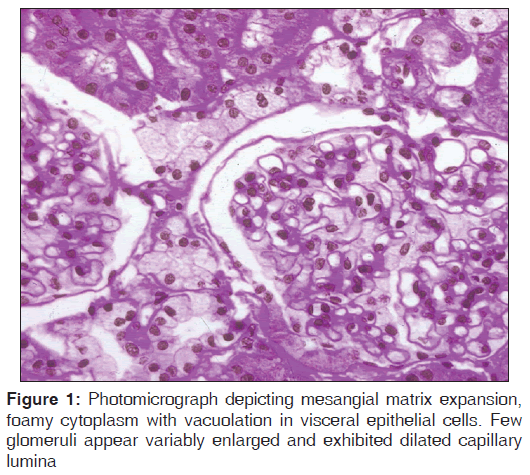
The biopsy contained renal medulla and cortical parenchyma containing up to 7 viable glomeruli [Figure 1]. One glomerulus showed global sclerosis. Remaining glomeruli appeared variably enlarged and exhibited dilated capillary lumina. Two glomeruli showed segmental sclerosis with a monolayer of visceral epithelial cells overlying the sclerosed segments. There was remarkable global enlargement of visceral epithelial cells which contained abundant vacuolated cytoplasm, with few cells containing cytoplasmic basophilic debris. Tubular atrophy and interstitial fibrosis involved about 15–20% of sampled cortex. Patchy dense interstitial inflammation and mild interstitial edema with a few foci of moderate tubulitis affecting the nonatrophic tubules were noted. Patchy loss of tubular brush borders with epithelial simplification and luminal granular casts were seen. Few atrophic tubules were dilated and contained luminal hyaline/proteinaceous casts whereas few tubular lumina contained red blood cells (RBC’s). Arteries appeared relatively unremarkable while arterioles showed focally marked hyalinosis. Focal RBC extravasation and interstitial inflammation were noted in the medullary parenchyma. Tissue for immunofluorescence showed up to 5 glomeruli, exhibiting segmental mesangial staining for IgM, and were negative for IgA, IgG, C3 and C1q.
The patient received pulse doses of methylprednisolone for cell‑mediated rejection and was continued on immunosuppressive regime of prednisone, tacrolimus and mycophenolate mofetil, and olmesartan for proteinuria. Serum creatinine initially improved to 1.7 mg/dl. However, subsequent testing revealed worsening serum creatinine to 2.7 mg/dl. In view of renal biopsy showing visceral epithelial cells containing abundant vacuolated cytoplasm, patient was evaluated for alpha‑galactosidase A enzyme activity. Levels of the enzyme was found markedly deficient in the recipient (7.00; enzyme normal limit >60). Diagnosis of FD was confirmed in the recipient. Further evaluation revealed normal electrocardiogram and normal echocardiography. There was mild acroparesthesias in the hands and feet of the recipient. The renal function of the donor continued to remain within normal limits (serum creatinine 0.8 mg/dl and urinary protein of <50 mg/24 h). The alpha‑galactosidase level of donor was normal on evaluation.
A pedigree analysis showed recipient was second in birth order and had four more brothers. A second sibling, another brother, fourth in birth order, who was evaluated for infertility was found to have raised serum creatinine (serum creatinine of 4 mg/dl) with proteinuria of 5.8 g/24 h. He was evaluated for alpha‑galactosidase activity which was found markedly decreased (12.10; normal enzyme activity level >60), and was diagnosed as FD with renal failure. The mother of the patient had died at around 55 years of age due to chronic kidney disease (CKD)/ESRD, the etiology of which was not known as she was not evaluated for it. The father is alive at the age of 90 years and is well. Three other brothers are not affected. His mother has three more siblings who are all alive and well with ages more than 75, but have not been evaluated for FD. The children of the patient are being evaluated for FD.
The patient is being planned for ERT. However, due to financial constraints has still not been started on ERT.
Case 2
A 16‑year‑old male presented in March 2002 with a fever of unknown origin and splenomegaly. The investigations revealed normal renal functions. USG of the abdomen revealed splenomegaly and echocardiography was normal. He was treated with antibiotics. The patient again presented in June, 2009 (now age 25 years) with fever, headache, loss of vision, and chest pain. Ophthalmological consultation revealed cloudy cornea and features of anterior ischemic optic neuropathy and patient was advised three doses of injectable methylprednisoslone. Magnetic resonance imaging of the brain done revealed nonspecific white matter hyperintensities in the left high parietal lobe. Echocardiography revealed severe left ventricular hypertrophy likely of the variant, hypertrophic cardiomyopathy. Urine examination revealed Alb ++ and 24 h urinary protein estimated 1 g/day. Serum creatinine was 2.7 mg/dl. The USG revealed bilateral small kidneys with borderline hepatosplenomegaly. In view of multisystem involvement, serum alpha‑galactosidase level was done which estimated 25 nmol/h/mg (normal >60 nmol/h/mg). There were mild acroparesthesias in the hands and feet. In March 2013, he was readmitted whereby his renal functions revealed creatinine of 9.36 mg/dl and USG revealed small‑sized kidneys. Recently, patient was admitted with seizures and advanced renal failure. Patient has been planned for renal replacement therapy (RRT). The pedigree analysis revealed that he had three brothers with complaints of decreased vision. They are planned for alpha‑galactosidase A levels and appropriate ERT.
Case 3
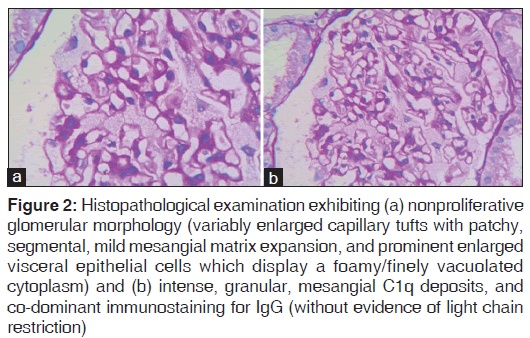
A 30‑year‑old female, historically normotensive, nondiabetic presented to us with 2 years history of pedal edema and periorbital swelling. On evaluation in 2011, her urine examination revealed Alb ‑ 3+, pus cells ‑ 18–20. Twenty‑four hours urinary protein had been 1.8 g/day. Kidney function tests revealed creatinine of 1.3 mg/dl. The right kidney was 8.8 × 2.9 in size and left kidney was 7.8 cm × 3.3 cm in size with normal hepatobillary and pelvic system. Her anti‑nuclear antibody titer (an ANA was 5.84 IU/ml, positive >1.2 IU/ml). The patient had been reluctant for renal biopsy. She had been treated with oral steroids and angiotensin receptor blocking agents. She presented to our department on November 2013. A full evaluation was sought. Her urine examination revealed Alb 2+, RBC 1–2, pus cells 4–5. Twenty‑four hours urinary protein estimation was 1.38 g/day. Serum creatinine was 1.85 mg/dl. ANA, anti‑dsDNA had been negative. Patient was subjected to renal biopsy. The examination of the renal tissue revealed up to seven glomeruli, one was globally sclerosed. Rest of the glomeruli displayed variably enlarged capillary tufts with patchy, segmental, and mild mesangial matrix expansion. Extracapillary cells (chiefly podocytes) were enlarged and displayed a foamy/finely vacuolated cytoplasm. No mesangial or endocapillary proliferation was noted and the peripheral glomerular capillary loops did not show significant thickening. There was no evidence of tuft necrosis/crescent formation or segmental sclerosis in the sampled glomeruli [Figure 2].
Figure 2: Histopathological examination exhibiting (a) nonproliferative glomerular morphology (variably enlarged capillary tufts with patchy, segmental, mild mesangial matrix expansion, and prominent enlarged visceral epithelial cells which display a foamy/finely vacuolated cytoplasm) and (b) intense, granular, mesangial C1q deposits, and co?dominant immunostaining for IgG (without evidence of light chain restriction)
Foci of tubular atrophy and interstitial fibrosis were seen involving approximately 15–20% of the sampled cortical parenchyma. The atrophic tubules contained inspissated hyaline casts. An associated, mild to moderate chronic interstitial inflammatory infiltrate was seen. The nonatrophic tubules showed patchy loss of brush borders with epithelial attenuation. The arteries sampled showed medial thickening and mild fibrointimal hyperplasia with elastic reduplication.
On differential immunofluorescence (DIF), sections included up to nine glomeruli exhibiting the following immunostaining features‑IgA negative, IgG ‑ 3+, IgM ‑ Trace, C3 ‑ negative, C1q ‑ 3+/4+, kappa light chains ‑ 1+/2+, lambda light chains ‑ 2+. The entire staining pattern above was predominantly mesangial. The glomeruli exhibited nonproliferative morphology with variably enlarged capillary tufts exhibiting patchy, segmental, mild mesangial matrix expansion, and prominent enlarged extracapillary cells (chiefly podocytes) which display a foamy/finely vacuolated cytoplasm.
Further special stains were performed on this biopsy. Periodic acid–schiff (PAS) stained, frozen‑sections of saline preserved tissue revealed intracytoplasmic PAS‑positive granules within the podocytes/parietal epithelial cells. On examination of unstained frozen‑sections under polarized light, these granules showed a Maltese cross pattern. With these DIF findings of non specific mesangial entrapment of complement and immunoglobulin, a possibility of renal involvement in a metabolic disorder viz., FD was suggested.
The pedigree analysis revealed that the patient had three brothers and one sister. Clinical examination showed angiokeratoma and corneal haziness in father and one of the brothers. Enzyme levels in all members had been sent.
The clinical characteristics of the studied population is shown in table 1.
| Patient characteristics | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Age | 45 | 16 | 30 | 24 | 38 |
| Sex | Male | Male | Female | Female | Male |
| Presenting feature | Known case | Fever, headache, | Anasarca, decreased | Parsthesia, edema legs, | CKD?IV with chest pain, |
| of ESRD | vision loss | urine output | weakness lower limbs | pain abdomen, vomiting | |
| Creatininine (mg/dl) | 2/1.7/2.7 | 2.7/9.3 | 1.3 | 3.0 | 2.8 |
| Proteinuria (g/day) | 4 | 1 | 1.8 | 2 | 2.5 |
| Alpha?galactosidase | 7 | 25 | 20 | ND | 18 |
| (nmol/h/mg) | |||||
| Renal biopsy | Conclusive | Conclusive | Conclusive | Conclusive | Conclusive |
| Etiology | FD | FD | FD | FD | FD |
| Family history | Positive for FD | Positive for FD | Not proven, | Positive | Not evaluated |
| under evaluation | |||||
| Other findings | Nil | Splenomegaly | Nil | Septal hypertrophy | Hepatosplenomegaly |
| ERT | Not given | Given | Given | Given | No |
| RRT | Yes | Yes | Not applicable | Not applicable | Planned |
| Outcome | Under observation | Improved | Improved | Under observation | Under observation |
ND: Not done, FD: Fabry?s disease, ERT: Enzyme replacement therapy, RRT: Renal replacement therapy, ESRD: End?stage renal disease, CKD: Chronic kidney disease
Table 1: Clinical characteristics of the studied population.
Discussion
FD is a rare lysosomal storage disease leading to progressive globotriaosylceramide (GL‑3) accumulation in multiple cell types and tissues with end organ damage. More than 500 different mutations have been identified in long arm of the X chromosome.
Renal dysfunction and ultimately ESRD occurs in classically affected males and in about 10–15% of female heterozygotes. FD is a rare cause of ESRD, accounting for 0.0167% of all causes of ESRD (according to US Renal Data System [USRDS] analysis),[2,4,5] 0.0188% (according to European Renal Association‑European Dialysis and Transplant Association).[6] Patients with FD are younger and more likely to be male and white. Mean age was 42 years and the majority of patients began RRT between the ages of 35 and 44 years in USRDS.[5]
Our case series is important because of following reasons: (1) FD is under‑diagnosed and screening of high‑risk groups is important for case finding and our observation highlights the same need (2) this case series highlights the importance of screening those patients for CKD who have unexplained renal failure (3) it highlights the need for pretransplant evaluation for FD.
Proteinuria is often the first sign of kidney involvement. Patient may present with proteinuria, isosthenuria and gradual deterioration of renal function. Development of azotaemia occurs in second through fourth decades.[1] In all the five patients, the diagnosis of FD was established after the diagnosis of renal disease. The diagnosis was established by enzyme level estimation and renal biopsy. In two patients, angiotensin receptor blockers (ARBs) were used for proteinuria to which patient responded well to. Recently, Tahir et al. found reduction in proteinuria after use of ARBs.[7]
Fabry nephropathy is one of the most severe manifestations of FD. It had been one of the unknown causes of morbidity and mortality before the widespread availability of dialysis and kidney transplantation. Like most aspects of FD, kidney disease is thought to result from GL‑3 accumulation in glomerular endothelial, mesangial and interstitial cells, podocytes, and renal vasculature. Progressive intracellular accumulation of GL‑3 is thought to cause glomerulosclerosis and interstitial fibrosis [8] as well as its urinary excretion together with other lipids.[9]
The clinical manifestations of FD in females tend to be less severe and present later than in males.[10] In this regard, they may develop albuminuria and progressive renal dysfunction leading to the need of RRT.[11,12]
Diagnosis may be presumptive based on observation of symptoms and laboratory findings with family history and medical pedigree. Definitive diagnosis is made by enzyme assay and gene mutation analysis or linkage analysis. Prenatal detection of affected males may be accomplished by demonstration of deficient α galactosidase A activity or the family’s specific gene mutation in chorionic villi obtained in the first trimester or in cultured amniocytes obtained by amniocentesis in the second trimester of pregnancy.[1]
Treatment includes ERT and RRT in the form of dialysis or kidney transplantation. The use of ARBs has been shown to be nephro‑protective in other proteinuric renal diseases, and could thus be important in FD as well.[13]
Kidney Disease Improving Global Outcomes guidelines suggest that in patients with CKD Stages 3–5, Vitamin D deficiency be corrected. Vitamin D can reduce proteinuria or albuminuria.[14]
Newer modalities under research include gene therapy substrate deprivation. Studies on ERT of FD patients on dialysis are warranted, as this may be the only hope as these patients have to improve survival on dialysis.
Although kidney transplantation is considered the optimal therapy for ESRD in suitable patients, it is not universally accepted as the ideal treatment for patients who have suffered ESRD from FD. Recent estimates of rate of transplantation of Fabry patients compared with matched controls appear to be similar, suggesting this practice may be changing.[2,5,6] Successful kidney transplantation in FD has been reported by several kidney transplant programs in the United States and Western Europe,[5,15,16] although there are case reports of recurrence of FD in the transplanted kidney.[17‑20] FD patients that initiate dialysis have a worse survival compared with non‑FD controls, even when one accounts for age.
Conclusion
Evaluation for FD is still not included in the regular protocols followed all over the world in pretransplant work‑up of donor or recipient. There is clearly a need to evaluate whether all kidney transplant recipients and living donors should undergo testing for FD prior to transplantation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- McGovern MM, Desnick RJ. Lysosomal storage diseases. In:Goldman L, editors. Cecil Textbook of Medicine. 21st ed. St.Louis: W.B. Saunders Company; 2000. p. 1104-7.
- Thadhani R, Wolf M, West ML, Tonelli M, Ruthazer R,Pastores GM, et al. Patients with Fabry disease on dialysis in the United States. Kidney Int 2002;61:249-55.
- Brady RO, Grabowski GA, Thadhani R. Fabry Disease: Review and New Perspectives. United states: Gardiner-Caldwell SynerMed; 2001. p. 1-8.
- Ojo A, Meier-Kriesche HU, Friedman G, Hanson J, Cibrik D, Leichtman A, et al. Excellent outcome of renal transplantation in patients with Fabry?s disease. Transplantation 2000;69:2337-9.
- Obrador GT, Ojo A, Thadhani R. End-stage renal disease in patients with Fabry disease. J Am Soc Nephrol 2002;13 Suppl 2:S144-6.
- Tsakiris D, Simpson HK, Jones EH, Briggs JD, Elinder CG, Mendel S, et al. Report on management of renale failure in Europe, XXVI, 1995. Rare diseases in renal replacement therapy in the ERA-EDTA Registry. Nephrol Dial Transplant 1996;11 Suppl 7:4-20.
- Tahir H, Jackson LL, Warnock DG. Antiproteinuric therapy and fabry nephropathy: Sustained reduction of proteinuria in patients receiving enzyme replacement therapy with agalsidase-beta. J Am Soc Nephrol 2007;18:2609-17.
- Alroy J, Sabnis S, Kopp JB. Renal pathology in Fabry disease. J Am Soc Nephrol 2002;13 Suppl 2:S134-8.
- Branton MH, Schiffmann R, Sabnis SG, Murray GJ, Quirk JM, Altarescu G, et al. Natural history of Fabry renal disease: Influence of alpha-galactosidase A activity and genetic mutations on clinical course. Medicine (Baltimore) 2002;81:122-38.
- Schiffmann R, Warnock DG, Banikazemi M, Bultas J, Linthorst GE, Packman S, et al. Fabry disease: Progression of nephropathy, and prevalence of cardiac and cerebrovascular events before enzyme replacement therapy. Nephrol Dial Transplant 2009;24:2102-11.
- Ortiz A, Oliveira JP, Waldek S, Warnock DG, Cianciaruso B, Wanner C; Fabry Registry. ephropathy in males and females with Fabry disease: Cross-sectional description of patients before treatment with enzyme replacement therapy. Nephrol Dial Transplant 2008;23:1600-7.
- Ortiz A, Cianciaruso B, Cizmarik M, Germain DP, Mignani R,Oliveira JP, et al. End-stage renal disease in patients with Fabry disease: Natural history data from the Fabry Registry.Nephrol Dial Transplant 2010;25:769-75.
- Batista EC, Carvalho LR, Casarini DE, Carmona AK, dos Santos EL, da Silva ED, et al. ACE activity is modulated by the enzyme a-galactosidase A. J Mol Med (Berl) 2011;89:65-74.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 2009;76:S1-130.
- Donati D, Novario R, Gastaldi L. Natural history and treatment of uremia secondary to Fabry?s disease: An European experience. Nephron 1987;46:353-9.
- Sheth KJ, Roth DA, Adams MB. Early renal failure in Fabry?s disease. Am J Kidney Dis 983;2:651-4.
- Popli S, Molnar ZV, Leehey DJ, Daugirdas JT, Roth DA, Adams MB, et al. Involvement of renal allograft by Fabry?s disease. Am J Nephrol 1987;7:316-8.
- Schweitzer EJ, Drachenberg CB, Bartlett ST. Living kidney donor and recipient evaluation in Fabry?s disease. Transplantation 1992;54:924-7.
- Kochar O, Wick MR, Kerr SE, Oglesbee D, Cathro HP. Unexpected Fabry disease in a renal allograft kidney: An underrecognized cause of poor allograft function. Ultrastruct Pathol 2011;35:92-6.
- Paull LS, Lipinski MJ, Wilson WG, Lipinski SE. Female with Fabry disease unknowingly donates affected kidney to sister: A call for pre-transplant genetic testing. JIMD Rep 2012;4:1-4.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.