Forced Expiratory Volume in 6 s (FEV6) and FEV1/FEV6 Values as a Viable Alternative for Forced Vital Capacity (FVC) and FEV1/FVC Values During Pregnancy in South East Nigeria: A Preliminary Study
- *Corresponding Author:
- Prof. Uchenna Nwagha
Departments of Physiology, and Obstetrics and Gynecology, College of Medicine, University of Nigeria, Enugu Campus, Nigeria.
E-mail: uchenna.nwagha@unn.edu.ng, uchenwagha@yahoo.com
Abstract
Background: Forced expiratory volume in 6 s (FEV6) and FEV1/FEV6 ratio have traditionally been used as a surrogate for forced vital capacity (FVC) and FEV1/FVC in the assessment of spirometric lung function in nonpregnant subjects. However, the existence of this relationship during pregnancy is yet to be ascertained. Aim: The aim of this study was to determine if FEV6 and FEV1/FEV6 can effectively be used instead of FVC and FEV1/FVC in the interpretation of lung function test during pregnancy. Subjects and Methods: This study was a descriptive cross‑sectional study carried out among 200 pregnant women who were recruited by using systematic random sampling during the period between April and October 2011. One hundred matched nonpregnant women served as control. A standard spirometer was used to determine the FVC, FEV6, FEV1/FVC, and FEV1/FEV6. Data analysis was done using SPSS version 11.0 (Chicago, IL, USA). Values were recorded as mean (standard deviation), and also median and interquartile ranges. The one‑way analysis of variance, Mann–Whitey U and the Kruskal–Wallis were used to test for significance where applicable. P <0.05 were considered to be significant. Results: All the values were within normal range, but the FVC and FEV6 decreased significantly while the FEV1/FVC and FEV1/FEV6 increased as pregnancy progressed. However, for first and last trimester, FVC differed significantly from FEV6. The values of the FVC were comparable to the values of FEV6. The FEV1/FVC and FEV1/FEV6 ratio were similar and well above the 0.7 cutoff for obstructive lung diseases. Conclusion: FEV6 requires a short exhalation time and can effectively be used in place of FVC in evaluation of lung function test during pregnancy. The FEV1/FEV6 may be applied as a proxy for FEV1/FVC in pregnant and nonpregnant women.
Keywords
FEV, FEV/FEV, FEV/FVC, Forced vital capacity, Pregnancy, South East Nigeria
Introduction
Spirometry is the most widely used pulmonary function test. It is effort-dependent and requires careful instruction, and the full cooperation of the test subject.[1] The American Thoracic Society (ATS)/European Respiratory Society (ERS) standards for the diagnosis and management of patients with chronic obstructive airway disease (COPD) recommend a fixed proportion of forced expiratory volume in 1 s and forced vital capacity (FEV1/FVC) of 0.7 as the cutoff.[2,3] A postbronchodilator FEV1/FVC ≤0.7 confirms the presence of airflow limitation that is not fully reversible.[2] In performing the FVC maneuver, the entire exhalation time can be prolonged and technically demanding in patients with severe airflow limitation.[1] Forced expiratory volume in 6 s (FEV6) as a surrogate for the FVC has recently been found to be admissible in nonpregnant clinical settings.[4,5] In addition, some studies have also indicated that FEV1/FEV6 ratio can conveniently be used as a valid substitute for FEV1/FVC in nonpregnant situations.[6,7]
Compared with measurements of FVC, using FEV6 reduces the test time and frustration,[8] and also reduces complications such as syncope.[9] Furthermore, in situations of poor expiratory effort, FVC is usually underestimated, as air flow toward the end of FVC is significantly reduced, making it a bit difficult for the spirometer to detect the flow.[10] Consequently, shorter spirometry maneuvers; allowing subjects to stop after 5, 6, and 7 s have been suggested.[11] Moreover, normal FEV1/FVC does not exclude airflow obstruction as a pattern of “pseudo-restriction” (concomitantly decreased FEV1 and FVC and therefore normal FEV1/FVC ratio) can occur if the subject cannot exhale long enough to clear the lungs to the residual volume.[11]
The reference values of FEV6, which is the volume in the spirometer at exactly 6 s after the maneuver have been determined, and indeed provided researchers with opportunity to use shorter FVC maneuvers during spirometry.[12] The study by Swanney et al., further demonstrated that using the FEV1/FEV6 to identify airway obstruction in 337 patients referred to a hospital-based pulmonary function laboratory resulted in low misclassification rate when compared with the traditional FEV1/FVC.[13] Indeed, it has been elucidated that the FEV1/FEV6 predicted the subsequent 5-year decline in FEV1, as well as the FEV1/FVC in 5887 adult smokers.[5] As the clamor for these viable alternatives generated momentum, population prediction equations for FEV1/FEV6 were determined in the US, Europe, and Asia.[8,12,14] A remarkably recent meta-analysis of a systematic review has unequivocally strengthened this relationship.[15]
Pregnancy, although a physiological process is associated with significant variation in lung function.[16] Furthermore, other changes that affect the maternal ability to perform strenuous efforts are also evident. The situation is even more precarious in developing countries, like in the study population, where nutritional deficiencies and maternal anemia are highly prevalent.[17] It may therefore be difficult for many pregnant women to put enough effort at the required period of 20 s to obtain reliable FVC and FEV1/FVC results. Consequently, it may be clinically expedient to use FEV6 as a replacement for FVC, and FEV1/FEV6 as a replacement for FEV1/FVC ratio in the interpretation of lung function test. The baseline values of these parameters to determine if they can complement each other are therefore vital since values are critically necessary to the administration of epidural anesthesia during labor, and specifically during cesarean section. Unfortunately, information that compares these parameters during pregnancy is difficult to obtain. As a result, in this study, the possibility of using FEV6 and FEV1/FEV6 ratio as a viable alternative to FVC and FEV1/FVC during the assessment of spirometric lung function in pregnancy is evaluated.
Subjects and Methods
This was a descriptive cross-sectional study carried out among 200 pregnant women, who were recruited using systematic random sampling between April and October 2011, at the antenatal care (ANC) and booking clinics of a University Teaching Hospital and two other secondary health care facilities in South, East Nigeria. The control subjects were 100 nonpregnant female employees working in the said institutions. The pregnant women and the control were matched for age, height, and socioeconomic status.
Ethical approval was obtained from the Ethics Committee of the University of Nigeria Teaching Hospital (UNTH), Enugu and informed written consent was obtained from the subjects. The ethical approval obtained was presented to the other institutions for ratification before commencement of the study.
Smokers and subjects who had worked or who work in dusty environments like coal mining or street cleaners and those with preexisting cardio-respiratory diseases such as asthma, COPD, congestive cardiac failure, and presence of obvious spinal deformities (scoliosis and kyphoscoliosis) were excluded. Those previously or currently treated for pulmonary tuberculosis, upper and lower respiratory tract infections, medications that alter lung function (e.g., bronchodilators and constrictors), acute malaria in pregnancy, preeclampsia, diabetes in pregnancy, febrile conditions, multiple pregnancy, chronic renal disease, sickle cell anemia, HIV positive patients, and other pregnancy complications (threatened abortion, antepartum hemorrhage, etc.) were also excluded from the study.
For optimal and reproducible results to be obtained, the tests were conducted between 9 am and 11 am on the study days. Those who consumed alcohol within 4 h of testing, those that did vigorous exercise within 30 min of testing or who were wearing clothes that substantially restrict chest and abdominal movement, those that ate a large meal within 2 h of testing or had chest or abdominal pain of any etiology or pain in the mouth or face that will be worsened by mouthpiece, dementia or confusional state and stress incontinence[18] were also excluded.
The minimum sample size was determined using the following formula:[19]
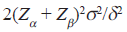
Thus, with Zα, which is the point of the normal distribution corresponding to the one-sided significance level = 1.65, Zβ, the one-sided % point of the normal distribution; corresponding to power is 80%, σ, the average population standard deviation of 0.80 (range 0.38-0.96), obtained from a previous study within the same reproductive age group),[20] and δ, the expected mean difference of approximately 0.56, the minimum sample size was calculated to be 100 (25 per group). However, due to the inherent anticipated difficulties of meeting the established criteria and also difficulties to be encountered when pregnant women are asked to exhale forcefully, 200 pregnant and 100 nonpregnant women, were recruited.
A pretested interviewer administered questionnaire patterned after the 1976 British MRC questionnaire on respiratory symptoms as modified by Pistelli et al.[21] was used to obtain the information directly from the subjects. English language combined with local language, where necessary was used in administering the questionnaire. Personal history, history of current pregnancy, past obstetric history, past medical history, family and social history, and review of systems was obtained. The gestational age was assessed from the last normal menstrual period, and findings were collaborated with symphysio-fundal height measurement and ultrasonography where applicable. Only those who were convinced of their last normal menstrual period were included. Trimester was defined as the 1st trimester (<14 weeks), 2nd trimester (14-27 weeks), and 3rd trimester (>27 weeks). The measurements for the control subjects were done on the 7th day of the last menstrual period after a negative blood pregnancy test. Complete physical and obstetric examinations were performed in each subject. All the baseline laboratory investigations for ANC were performed. These include hemoglobin, blood group and genotype, urinalysis, fasting blood sugar and 2 h postprandial. Other investigations include; screening for HIV, hepatitis B surface antigen, and screening for syphilis.
Measurement of anthropometric indices
The weights were measured to the nearest 0.1 kg using a standard weighing scale (Stadiometer, Seca, Model 220, Germany). The heights were measured to the nearest 0.1 cm, without shoes, with the feet together, standing as long as possible with the eyes level and looking straight ahead, using a standard measuring stick located in the same equipment. The same instrument was used for all the measurements after due calibration before individual measurement. The height was converted to meters and the body mass index (BMI) was calculated by weight in kg with the height in m2 and expressed as kg/m2.
Spirometry
A standard Spirometer (Micro lab ML3500 MK8, Cardinal Health Germany 234 GMBH) with disposable mouth piece was used. The principal investigator, who was trained in the pulmonary medicine unit of the UNTH on the use of spirometer, was in charge of the equipment and the method. The ambient temperature, barometric pressure and time of the day of the measurement were recorded. It was ensured that time of the day was within 2 h of the earlier study periods. Subjects were counseled and given instructions and then personal demonstration of how best to blow the spirometer. Subjects were relaxed, dentures removed, and tight fitting clothes loosened. After measuring the basal respiratory rate, each woman was told to sit upright in a straight backed chair, with her belt loosened. She was then asked to breathe normally for about 20 s, then breathes in as hard as possible and holds the breath. She then applies her lips around the mouthpiece of the spirometer firmly and breathes out as quickly and as forcibly as possible into the spirometer. It was ensured that there is no leakage of air from the mouth piece. The procedure was repeated when any leakage was observed. The equipment automatically selects the best out of three maneuvers when the ATS/ERS guidelines must have been met (three good blows with values within 5% or 0.15 L (150 ml). Analyses of data were done using Statistical Package for Social Sciences (SPSS version 11, Chicago, IL USA), graph pad prism version 5.02 and graph pad prism state mate version 2.00. The D’Agostino and Pearson omnibus normality tests were performed, and the lung function data did not obey Gaussian distribution (not normally distributed). Consequently, the FVC, FEV6, FEV1/FVC, and FEV1/FEV6 were recorded as percentages, median (interquartile range [IQR]), minimum and maximum, while the sociodemographic data were recorded as mean (SD). The one-way analysis of variance, Mann–Whitney U, and the Kruskal–Wallis, with the honestly significant posthoc multiple comparisons were used to analyze data. The respiratory function indices in pregnancy were compared with the values found in the matched controls and duration of pregnancy grouped as trimesters.
Results
Of the 300 subjectes recruited, 172 (40 control, 30 1st trimester, 48 2nd trimester, and 54 3rd trimester) met the ERS/ATS quality control criteria, and were thus included in the analyses. Some socio-anthropometric characteristics of the subjects are represented in Table 1. All the subjects had formal education. Majority had secondary education 54/172 (31.4%), diploma and other certificates other than university after their secondary school constituted, 52/172 (30.2%), 51/172 (29.7%) had university education, while only (15/173) 8.7% had primary education. Most subjects were house wives, civil servants, and teachers constituting (44/172) 25.6%, 43/172 (25.0%), and 41/172 (23.8%), respectively. Others were nurses 14/172 (8.1%), students and traders 9/172 (5.2%). Lawyers, bankers, hairdressers, and apprentices were approximately represented with 3/172 (1.8%) each.
| Variables | Control | 1st trimester | 2nd trimester | 3rd trimester | Significant |
|---|---|---|---|---|---|
| Age (years) | 30.75 (5.45) | 30.07 (4.41) | 31.50 (3.76) | 29.44 (5.08) | 0.16ns |
| Parity | 2.95 (1.48) | 1.83 (1.64) | 2.25 (2.15) | 1.67 (1.67) | <0.01* |
| Height (m) | 1.65 (0.06) | 1.65 (0.05) | 1.66 (0.06) | 1.66 (0.07) | 0.50ns |
| Weight (kg) | 71.07 (9.42) | 74.59 (9.87) | 75.75 (9.81) | 78.61 (13.62) | 0.01* |
| BMI (kg/m2) | 27.29 (2.82) | 27.24 (4.18) | 28.62 (3.64) | 28.83 (3.22) | 0.06ns |
m: Meter, kg: Kilogram, BMI: Body mass index, ns: Non-significant. P>0.05, *P<0.05 (ANOVA)
Table 1: Anthropometric characteristics of the subjects
The mean age of the subjects were not significantly different (P = 0.16). The highest parity occurred in the control subjects, 2.95 (1.48) while the least parity was in the 3rd trimester 1.67 (1.67) (P < 0.01). The differences in weight were significant (P = 0.01), while the differences in height were not statistically significant, (P = 0.50). Although, the BMI increased as pregnancy progressed, these were not statistically significant (P = 0.06).
The values of the FVC and FEV6 are shown in Table 2. The FVC values are within normal range, but decreased significantly as pregnancy progressed (P < 0.001), with the largest decrease occurring in the 2nd trimester. This is mainly due to differences between control versus 3rd trimester and 1st versus 2nd and 3rd trimesters, respectively. The FEV6 was also within normal range but exhibited a slight significant reduction with increasing gestational age, P = 0.04 (more in the 2nd trimester), although no real differences existed between the groups (posthoc analysis).
| Control (L) | 1st trimester (L) | 2nd trimester (L) | 3rd trimester (L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FVC | FEV6 | FVC | FEV6 | FVC | FEV6 | FVC | FEV6 | ||||
| Minimum | 1.94 | 1.94 | 2.09 | 2.11 | 1.90 | 1.90 | 1.66 | 1.66 | |||
| 25th percentile | 2.38 | 2.38 | 2.78 | 2.37 | 2.24 | 2.24 | 2.17 | 2.19 | |||
| Median | 2.91 | 2.92 | 2.98 | 2.75 | 2.49 | 2.49 | 2.74 | 2.86 | |||
| 75th percentile | 3.14 | 3.14 | 3.28 | 3.25 | 2.92 | 2.92 | 2.93 | 2.98 | |||
| Maximum | 4.55 | 4.51 | 3.30 | 3.43 | 3.17 | 3.17 | 3.32 | 3.32 | |||
The FVC values are within normal range, but decreased significantly as pregnancy progressed (P<0.001). The FEV6 was also within normal range but exhibited a slight significant reduction with increasing gestational age, P=0.04 Kruskal-Wallis). The FVC values are within normal range, but decreased significantly as pregnancy progressed (P<0.001). The FEV6 was also within normal range but exhibited a slight significant reduction with increasing gestational age, P=0.04 (Kruskal-Wallis)
Table 2: FVC and FEV6 (L) values during pregnancy
Table 3 presents the data for FEV1/FEV6 and FEV1/FVC. The ratio of FEV1/FEV6 was within normal range, but showed a statistically significant increase (P < 0.001) as pregnancy progressed. The FEV1/FVC was also within normal range. The value increased significantly in pregnancy (P < 0.001). This was more pronounced in the 2nd trimester. For the FEV1/FEV6, the difference was primarily due to the differences between control versus 2nd trimester, 1st versus 2nd trimester and 3rd trimester, respectively. However, for the FEV1/FVC, the control versus 1st, and the 2nd versus 3rd trimester did not contribute to the changes observed (posthoc analysis). Table 4 represents Mann-Whitney U inferential statistics FEV6 versus FVC and FEV1/FEV6 versus FEV1/FVC.
\| Control (%) | 1st trimester (%) | 2nd trimester (%) | 3rd trimester (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FEV1/FVC | FEV1/FEV6* | FEV1/FVC | FEV1/FEV6 | FEV1/FVC | FEV1/FEV6 | FEV1/FVC | FEV1/FEV6 | ||||
| Minimum | 85.00 | 85.00 | 84.00 | 85.00 | 84.00 | 83.00 | 81.00 | 83.00 | |||
| 25th percentile | 85.00 | 85.00 | 86.00 | 86.00 | 88.20 | 88.25 | 86.00 | 89.00 | |||
| Median | 86.50 | 86.00 | 86.00 | 88.00 | 91.00 | 91.00 | 89.00 | 92.00 | |||
| 75th percentile | 89.00 | 89.00 | 88.00 | 89.00 | 92.00 | 92.00 | 92.00 | 93.00 | |||
| Maximum | 93.00 | 94.00 | 89.00 | 90.00 | 100.00 | 100.00 | 93.00 | 100.00 | |||
The ratio of FEV1/FEV6 was within normal range, but showed a statistically significant increase (P<0.001) as pregnancy progressed. The FEV1/FVC was also within normal range. The value increased significantly in pregnancy (P<0.001), but more pronounced in the second trimester.(Kruskal-Wallis)
Table 3: FEV1/FVC, and FEV1/FEV6) values during pregnancy
| Number | FEV6 versus FVC (L) |
FEV1/FEV6 versus FEV1/FVC (%) |
Comments | |
|---|---|---|---|---|
| Control | 40 | P=0.91 | P=0.99 | *Significant |
| 1st trimester | 30 | P=0.17 | P<0.01* | |
| 2nd trimester | 48 | P=0.98 | P=0.95 | |
| 3rd trimester | 54 | P=0.66 | P<0.01* |
FVC: Forced vital capacity, FEV6: Forced expiratory volume in 6 s, *Significant
Table 4: Mann-Whitney U inferential statistics FEV6 versus FVC and FEV1/FEV6 versus FEV1/FVC
Discussion
In this study, all the subjects were within the reproductive age group, and there were no significant differences between the age groups. The fact that the highest parity occurred in the control group represents a selection bias as most of those who met the selection criteria for the control population had completed their families. The height did not show any significant difference, and although, the weight understandable increased significantly, the BMI showed an insignificant increase. The amount of weight gained during a single pregnancy varies among women. The overall pregnancy weight gain for women starting pregnancy at a normal weight, with a BMI of 18.5-24.9 range from 11.4 to 15.9 kg/m2.[22]
All the subjects had formal education. Indeed the majority had secondary education as the least qualification. Again this represents a selection bias as most of the illiterates initially recruited could not understand the instruction to be followed during spirometry. This, lack of understanding of the procedure also led to the inability of the most of the recruited subjects to meet the ERS criteria on quality control and were eventually dropped from the study with a dropout rate of 42.6%. Understandably, majority of the subjects were civil servants and house wives. Although Enugu has always been known as a city of civil servants, majority of the professionals we recruited abandoned the procedure due to time factor as each procedure took a minimum of 30 min to obtain a reliable result. These could not have affected our findings as the variables are not strongly associated with changes in lung function.
The FVC and the FEV1 were within normal range in both the nonpregnant and pregnant subjects. However, the values decreased significantly as pregnancy progressed. Although some earlier studies did not reveal any significant change in these parameters during pregnancy,[23] a more recent study agreed with our findings.[24] The reduction in these values may be due to a comparative decrease in the negativity of the intrapleural pressure occasioned by an upward displacement of the diaphragm by the enlarging uterus. Another likely reason is the reduction in alveolar PCO2, caused by pregnancy associated hyperventilation, causing some degree of bronchoconstriction. Furthermore, poor nutrition especially micronutrients in pregnancy in our environment as earlier reported may be a contributory factor.[17]
The FEV1/FVC increased significantly as pregnancy progressed. In a study in northern India, this variable also increased, but not significantly.[18] FEV1 did not decrease as much as FVC hence a rise in the FEV1/FVC ratio. FEV1/FEV6 ratio also increased as gestational age increased. Again, this may be due to a comparative decrease in the negativity of the intrapleural pressure occasioned by an upward displacement of the diaphragm by the enlarging uterus. The reduced, but normal values for FVC and FEV1 with higher, but normal values for FEV1/FVC is a clear indication that physiological restriction occurs during pregnancy.[2]
In this study, FVC was similar to FEV6 in all subjects and this agreed with findings from other studies.[5,6] However, in the 1st trimester and 3rd trimesters, FEV1/FVC differed significantly from FEV1/FEV6 while in nonpregnant, and the 2nd trimester, FEV1/FVC was similar to FEV1/FEV6 but all the values were normal values of 80% and above. During the 2nd trimester, the woman is accustomed to the changes of pregnancy, and this may improve their effort making during spirometry. In the 3rd trimester, the displacement of the diaphragm by the enlarged uterus presents another obstacle in respiratory movement, which has variable tolerability by the subjects.
The implication of these findings, in the interpretation of spirometry findings during pregnancy is that the FEV6 can conveniently replace FVC in the 2nd trimester in our setting. This is of critical value in the diagnosis of obstructive airway disease during pregnancy, and also in the determination of lung function in normal pregnant women undergoing spinal and epidural analgesia during labor and cesarean section. Equally, we can posit that FEV1/FEV6 can be used as a proxy to FEV1/FVC ratio during pregnancy since the values are well above the 70% cutoff despite the statistical significant difference between the two in the 1st and 3rd trimester of pregnancy.
This preliminary study forms a pivot that provides a base line information as to the possibility of reducing the expiration time during spirometry in pregnant women, who are already overwhelmed by the heavy burden of physical and micronutrients depletion, especially in limited resource settings.[17] This study is however without limitations, this work would have been more robust if a community-based, multicenter research is undertaken, recruiting the subjects in the 1st trimester and following them up until 6 weeks after delivery. Small size of the control subjects compared to the tests subjects may have affected some inferences drawn. Larger sample size would be required to enable the production of prediction equation during pregnancy in the study environment. In addition, the assessment of pulmonary gases and endocrine reproductive hormones to determine the relationship of these hormones with pulmonary function should be considered in future.
FEV6 is a viable alternative to FVC and therefore can be used as a surrogate in the determination of lung function in pregnancy. However, for FEV1/FVC and FEV1/FEV6 ratio, we should be cautious because of the apparent statistical differences between them though the values are well above the normal ranges and above the cutoff of 0.7 [2,3] for the diagnoses of obstructive airway diseases.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Akpinar-Elci M, Fedan KB, Enright PL. FEV6 as a surrogate for FVC in detecting airways obstruction and restriction in the workplace. Eur Respir J 2006;27:374-7.
- Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26:948-68.
- Celli BR, MacNee W, ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: A summary of the ATS/ERS position paper. Eur Respir J 2004;23:932-46.
- Townsend MC. ACOEM position statement. Spirometry in the occupational setting. American College of Occupational and Environmental Medicine. J Occup Environ Med 2000;42:228-45.
- Enright RL, Connett JE, Bailey WC. The FEV1/FEV6 predicts lung function decline in adult smokers. Respir Med 2002;96:444-9.
- Vandevoorde J, Verbanck S, Schuermans D, Kartounian J, Vincken W. FEV1/FEV6 and FEV6 as an alternative for FEV1/FVC and FVC in the spirometric detection of airway obstruction and restriction. Chest 2005;127:1560-4.
- Melbye H, Medbø A, Crockett A. The FEV1/FEV6 ratio is a good substitute for the FEV1/FVC ratio in the elderly. Prim Care Respir J 2006;15:294-8.
- Pingul EM, de Guia TS, Ayuyao FG. FEV1/FEV6 vs FEV1/FVC in the spirometric diagnosis of airways obstruction among Asians. Chest 2007;132:491c-2.
- Hansen JE, Sun XG, Wasserman K. Should forced expiratory volume in six seconds replace forced vital capacity to detect airway obstruction? Eur Respir J 2006;27: 1244-50.
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38.
- Stănescu D, Veriter C. A normal FEV1/VC ratio does not exclude airway obstruction. Respiration 2004;71:348-52.
- Hankinson JL, Crapo RO, Jensen RL. Spirometric reference values for the 6-s FVC maneuver. Chest 2003;124:1805-11.
- Swanney MP, Jensen RL, Crichton DA, Beckert LE, Cardno LA, Crapo RO. FEV (6) is an acceptable surrogate for FVC in the spirometric diagnosis of airway obstruction and restriction. Am J Respir Crit Care Med 2000;162:917-9.
- García-Río F, Pino JM, Dorgham A, Alonso A, Villamor J. Spirometric reference equations for European females and males aged 65-85 yrs. Eur Respir J 2004;24:397-405.
- Jing JY, Huang TC, Cui W, Xu F, Shen HH. Should FEV1/FEV6 replace FEV1/FVC ratio to detect airway obstruction? A metaanalysis. Chest 2009;135:991-8.
- Wise RA, Polito AJ, Krishnan V. Respiratory physiologic changes in pregnancy. Immunol Allergy Clin North Am 2006;26:1-12.
- Ogbodo S, Nwagha U, Okaka A, Okeke A, Chukwurah F, Ezeonu P. Low levels of some nutritional parameters of pregnant women in a rural community of South East Nigeria: Implications for the attainment of the millennium developmental goal. Ann Med Health Sci Res 2012;2:49-55.
- Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, et al. General considerations for lung function testing. Eur Respir J 2005;26:153-61.
- Campbell MJ, Machin D. Medical Statistics: A Commonsense Approach. Vol. 2. New York: John Wiley and Sons; 1996. p. 156-7.
- Nku CO, Peters EJ, Eshiet AI, Bisong SA, Osim EE. Prediction formulae for lung function parameters in females of south eastern Nigeria. Niger J Physiol Sci 2006;21:43-7.
- Pistelli F, Viegi G, Carrozzi L, Rönmark E, Lundbäck B, Giuntini G. Appendix3: Compendium of respiratory standard questionnaires for adults (CORSQ). Eur Respir Rev 2001;11:118-43.
- Broughton-Pipkin F. Maternal physiology. In: Edmonds DK, editor. Dewhurst Textbook of Obstetrics and Gynaecology. 7th ed. Oxford: Blackwell Publishing Oxford; 2007. p. 10-8.
- McAuliffe F, Kametas N, Costello J, Rafferty GF, Greenough A, Nicolaides K. Respiratory function in singleton and twin pregnancy. BJOG 2002;109:765-9.
- Neeraj, Sodhi C, Pramod J, Singh J, Kaur V. Effect of advanced uncomplicated pregnancy on pulmonary function parameters of North Indian subjects. Indian J Physiol Pharmacol 2010;54:69-72.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.