Impact of Chemotherapy on Anti-Müllerian Hormone (AMH) and other Circulating Hormones in Carcinoma Breast
Citation: Bala J, et al. Impact of Chemotherapy on Anti-Müllerian Hormone (AMH) and other Circulating Hormones in Carcinoma Breast. Ann Med Health Sci Res. 2017; 7: 47-51
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Introduction: Incidence of breast cancer in women varies with age, mammary gland mass and exposure to endogenous and exogenous hormones. Breast cancer grows within a hormonal milieu which plays an important role in its development. Aim: The present study was planned to assess circulating hormone (serum estrogen (E2), progesterone, and testosterone, FSH, LH and AMH) levels in breast cancer patients and to elucidate the influence of chemotherapy on these hormones by comparing them with age matched healthy control women. Materials and Methods: This prospective study was conducted in the Department of Biochemistry in collaboration with Department of Radiotherapy in PGIMS, Rohtak. The subjects were divided into three groups: Group I (control group): Healthy age matched female volunteers with no prior history of breast cancer (n=30), Group II (study group): Newly diagnosed confirmed cases of breast cancer (n=30) and Group III (study group): Postchemotherapy group (n=30; Group II patients who had undergone chemotherapy). Serum Estrogen (E2), Progesterone, Testosterone, FSH, LH and AMH levels were estimated in healthy controls and patients before and after chemotherapy. Result: In the present study, E2 levels were significantly decreased while progesterone levels were significantly increased in group II and group III when compared with group I (p<0.001 and p<0.001 respectively). There was no significant change in Testosterone, FSH and LH levels in between study group (group II and group III) as compared with group I (p>0.05). AMH levels were also significantly decreased in both group II and group III when compared with group I (p<0.001). Conclusion: Present study concluded that AMH could be a valuable marker in these patients as it provides the exact knowledge of time by which the damage to granulosa cells and growing follicles occurs resulting in decreased ovarian reserve (OR); this is of great importance for the decision regarding the reproductive planning and optimal adjuvant hormonal treatment for women.
Keywords
Breast cancer; FSH; AMH; Ovarian reserve; Chemotherapy
Introduction
Breast cancer is malignant proliferation of epithelial cells lining the ducts or lobules of the breast. [1] Incidence of breast cancer in women varies with age, mammary gland mass, exposure to endogenous and exogenous hormones and international variation in incidence of breast cancer may be due to hormonal carcinogenesis. [2] When women develop breast cancer, the tumour grows within a hormonal milieu which plays a role in its development. [3]
Women’s Health Initiative (WHI) trial showed that conjugated equine estrogens plus progestins increased the risk of breast cancer. Hormone replacement therapy (HRT) for 6 to 7 years nearly doubled the risk of breast cancer. HRT in women previously diagnosed with breast cancer increases recurrence rates. [2]
Ovarian function has impact on cancer outcome, cancer treatment decisions, bone health and fertility issues. Some cancer treatments can damage the granulosa cells and decreases finite ovarian reserve (OR) in young patients. It is therefore important to understand how ovarian function is measured in cancer survivors. [4] Mechanism by which chemotherapeutic drugs affects ovarian function is not clearly known. Alkylating agents such as cyclophosphamide could lead to ovarian atrophy and stromal fibrosis, depletion of the primordial follicle stockpile, and to reduced ovarian weight, resulting in ovarian dysfunction. [5] Alkylating agents covalently bind an alkyl group to the DNA molecule and inhibits replication. In the ovaries they directly injure primordial oocytes, deplete follicles and damage the ovarian vasculature so that the follicles cannot grow. [6,7] Their destructive effect is dose-dependent and varies with the age and developmental maturity of the patient at the time of initiation of chemotherapy, with older women more likely to be left infertile after treatment. [8] Also, patient’s age correlates with the probability of ovarian damage or inversely with ovarian resistance to chemotherapy. Young women having more primordial oocytes, face a sharp reduction of their OR after chemotherapy. Still, younger patients show a lower rate of ovarian toxicity than older women. [9] Studies had reported presence or absence of menses as a measure of ovarian function. Since women can have residual ovarian function in the absence of menses and other factors such as adjuvant endocrine therapy can influence menses, the impact of chemotherapy on long-term ovarian, function is not well known. [10]
Postchemotherapy ovarian function recovery has implications for planning for individual patients for maintaining fertility; for earlier implementation of preventive measures related to menopause such as osteoporosis and coronary artery disease, for choice of adjuvant endocrine therapy for hormone receptorpositive (HR-positive) breast cancer. [10,11]
Currently, the primary tool and gold standard for assessing post-chemotherapy ovarian function is menstruation. There is a need for valid tools for measuring and predicting reproductive function in this population. Hence the present study was planned to assess AMH levels along with other circulating hormone in breast cancer patients to elucidate the still unknown influence of chemotherapy on these hormones.
Aim
This study was planned to assess AMH and other circulating hormone levels in breast cancer patients and to compare these levels with healthy age and sex matched controls.
Material and Method
This prospective study was conducted during 2013-2014 in the Department of Biochemistry in collaboration with Department of Radiotherapy in PGIMS, Rohtak. This study was approved by Ethics Review Committee of the institute. All patients were recruited after informed consent. The study included purposive sampling.
In this study, female breast cancer patients belonging to the age group of 30-70 were enrolled. Healthy controls were taken randomly from Out Patient Department. The subjects were divided into three groups:
• Group I (control group): Healthy age matched female volunteers with no prior history of breast cancer (n=30)
• Group II (study group): Newly diagnosed confirmed cases of breast cancer (n=30)
• Group III (study group): Postchemotherapy group (n=30; Group II patients who had undergone chemotherapy)
Females on oral contraceptive pills / hormone therapy or drugs affecting hormone levels were excluded from the study.
Staging of breast carcinoma was done according to TNM staging by the department of Radiotherapy. All patients were surgically managed (Modified Radical Mastectomy). Anticancer regimen 5-FU, Epirubicin, Cyclophosphamide (FEC) given for four cycles followed by four cycles of docitaxel. Each cycle followed by the next after a period of 3 weeks.
Five milliliters of venous blood sample was collected aseptically from anticubital vein from all the subjects. Estrogen (Estradiol, E2), [12] Progesterone, [13] Testosterone, [14] FSH [15] and LH [15] were estimated by a chemiluminisence method on ADVIA Centaur CP system from Siemens. Antimüllerian hormone (AMH) estimation done by a sandwich enzyme Linked- Immuno-Sorbent Assay (ELISA). [16]
An antibody specific for human AMH is coated onto the wells of the microtiter plate. Samples 0.1 ml and standards 0.1 ml of human AMH are pipetted into the wells for binding to the coated antibody. Seal the plate with the cover and incubate it at 37°C. After washing procedure to remove unbound compounds, an enzyme-linked antibody specific for human AMH (0.1 ml) is added to the wells and incubate the plate at 37°C. Following a wash to remove any unbound antibody-enzyme reagent, a substrate solution (0.1 ml) is added to the wells and adds 0.09 ml of prepared TMB colour developing agent into each well and incubate plate at 37°C away from light. Add 0.1 ml of prepared TMB stop solution into each well. The colour changes into yellow immediately. The intensity of the colour is measured. The magnitude for the absorbance for this developed colour is proportional to the amount of human AMH.
Result was expressed as mean values + Standard deviation. Analysis of Variance (ANOVA) and student‘t’ test was applied. Data were considered to be significant if p< 0.05 and highly significant with p< 0.001. SPSS, version 17.0 was used in the analysis (SPSS Inc. Released 2008. SPSS Statics for Windows, version 17.0. Chicago: SPSS Inc.).
Results
Table 1 shows the routine investigations of the group I (control group) and study group (Group II). The age in the study group (Group II and III) varied from 22-70 years having the mean age + SD as 49.30 + 11.35 years as compared to 47.63 + 11.88 years in the control group (Group I) also the age varied from 22-70 years. Out of 30 patients in the study group (Group II and III), 3 (10%) patients gave the family history of carcinoma breast in one of their close relatives whereas none in the control group (Group I) gave such history. 20% of the subjects in the study group (Group II and III) gave the history of smoking compared to 10% in the control group (Group I).
| Parameters | Group I (control group; n=30) | Group II (study group; n=30) |
|---|---|---|
| Mean age | 47.63 ± 11.88 | 49.30 ± 11.35 |
| Mean BMI | 27.05 ± 3.03 | 29.23 ± 2.68* |
| Mean Hemoglobin (g/dl) | 11.23 ± 1.46 | 10.5 ± 1.29 |
| Mean TLC (cells/cu.mm) | 6880 ± 1590.57 | 7296.66 ± 2516.9 |
| Blood urea (mg/dl) | 27.1 ± 5.72 | 32.1 ± 9.33* |
| AST/SGOT (U/L) | 33.46 ± 7.28 | 39.4 ± 22.58 |
| ALT/SGPT (U/L) | 33.0 ± 7.12 | 39.93 ± 18.24 |
| Serum alkaline phosphatase (U/L) | 94.0 ±10.65 | 167.1 ±154.1* |
| Serum creatinine (mg/dl) | 0.81 ± 0.11 | 0.88 ± 0.15 |
| Serum uric acid (mg/dl) | 4.27 ± 0.83 | 4.88 ± 1.22* |
Table 1: Routine investigations of group II (mean ± SD).
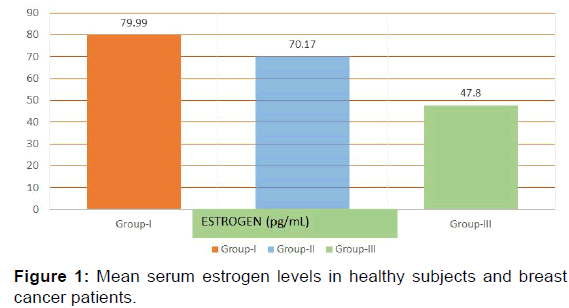
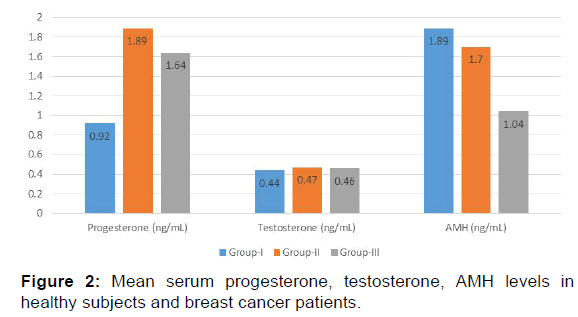
In the present study E2 levels were significantly decreased in group II (70.17+23.84 pg/mL) and group III (47.8+26.29 pg/ mL) when compared with the group I (79.99+26.79 pg/mL) [p< 0.001, Table 2, Figure 1]. Progesterone showed a significant increase in levels in group II (1.89+0.61 ng/mL) and group III (1.64+0.49 ng/mL) as compared with control group (group I) (0.92+0.39 ng/mL) [p< 0.001, Table 2, Figure 2] while testosterone showed non-significant changes in all the three groups; group I (0.44+0.10 ng/mL), group II (0.47+0.14 ng/mL) and group III (0.46+0.12 ng/mL) [p>0.05, Table 2, Figure 2].
| Parameters | Group-I | Group-II | Group-III | p-value |
|---|---|---|---|---|
| Estrogen (pg/mL) | 79.99 ± 26.79 | 70.17 ± 23.84 | 47.82 ± 26.29 | <0.001 |
| Progesterone (ng/mL) | 0.92 ± 0.39 | 1.89 ± 0.61 | 1.64 ± 0.49 | <0.001 |
| Testosterone (ng/mL) | 0.44 ± 0.10 | 0.47 ± 0.14 | 0.46 ± 0.12 | >0.05 |
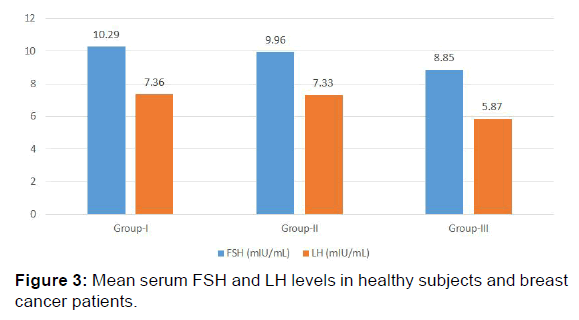
| FSH (mIU/mL) | 10.29 ± 3.31 | 9.96 ± 2.71 | 8.85 ± 4.21 | >0.05 |
| LH (mIU/mL) | 7.36 ± 3.05 | 7.33 ± 3.10 | 5.87 ± 2.26 | >0.05 |
| AMH (ng/mL) | 1.89 ± 0.34 | 1.70 ± 0.47 | 1.04 ± 0.26 | <0.001 |
Table 2: Mean serum values of estrogen, progesterone, testosterone, FSH, LH and AMH.
Mean FSH and LH levels showed no significant change in group II and group III when compared to control group (group I). [(9.96+2.71 mIU/mL, 8.85+4.21 mIU/mL, 10.29+3.31 mIU/mL and 7.33+3.10 mIU/mL, 5.87+2.26 mIU/mL, 7.36+3.05 mIU/ mL respectively) [p>0.05, Table 2, Figure 3]. The results of the present study indicate a significant decrease in AMH levels in both group II and group III as compared to group I (1.70+0.47 ng/mL, 1.04+0.26 ng/mL, 1.89+0.34 ng/mL respectively) [p< 0.001, Table 2 and Figure 2].
Discussion
Endocrine-associated risk factors are often related with an increased incidence of breast cancer in women. Circulating estrogen blood levels were seen positively associated with disease progression. [17] Increased risk were also seen with higher-than-normal blood levels of androstenedione and testosterone. Androgens can be directly converted by aromatase to the estrogens namely estrone and estradiol, respectively. [18] Elevated serum levels of testosterone remained associated with breast cancer in both premenopausal and postmenopausal women. [19]
In the present study, E2 levels were significantly decreased [p< 0.001, Table 2, Figure 1] while progesterone levels were significantly increased in group II and group III when compared with group I [p< 0.001, Table 2, Figure 2]. There was no significant change in Testosterone, FSH and LH levels in between study group (group II and group III) as compared with group I [p>0.05, Table 2, Figure 2 and Figure 3 respectively]. AMH levels were also significantly decreased in both group II and group III when compared with group I [p< 0.001, Table 2, Figure 2].
In breast tissue various progesterone metabolites are produced which act as independently active hormones functioning as cancer-promoting or -inhibiting regulatory agents. The maintenance of normalcy or progression to neoplasia depends on the ratios of pro- to anti-cancer progesterone metabolites in the local breast tissue. [20] Metabolic syndrome was related with elevated serum testosterone level and this had strong association with breast cancer progression. [21]
In the present study, FSH and LH levels showed no significant change in group II and group III when compared to group I. [p>0.05, Table 1, Figure 3].
Levels of FSH combined with E2 levels are often used to define the OR of women both before and after chemotherapy. FSH stimulates small antral follicular growth and causes selection of follicle having FSH receptors to form dominant pre-ovulatory follicle. [22,23] Follicular cells of granulosa cells (GC) produces E2 which in turn exerts a negative feedback at level of pituitary and hypothalamus resulting in decreased FSH levels. As the number of follicles decline with increasing age, E2 levels will also fall. Consequently FSH levels will rise termed as monotropic rise in FSH levels is the latest hormonal event preceding menopause. [24-26] In premenopausal women amenorrhea induced by chemotherapy may result in increased FSH levels which can be temporary and not always associated with absence of folliculogenesis in the future. [26] E2 is mainly secreted by the late antral follicle and the ensuing corpus luteum, controlled by FSH and LH. [27] Once the ovarian secretion of E2 is absent, the blood levels of E2 depend predominantly on peripheral conversion of androstenedione–testosterone to E2.24
Hypothalamo-pituitary-ovarian axis is often disturbed by chemotherapy and hormonal therapy, especially in premenopausal women. In postmenopausal women hormonal levels in serum can also be affected by chemotherapy, mainly by serum estrogen levels. This is important in patients with hormonal dependent tumors who need chemotherapy with respect to other evaluated risk factors. The mechanism of changes in the levels of these hormones could be due to impact of cytostatic drugs on the production these hormones in alternative tissues i.e., fat, breast and other or on process of regulation (FSH, LH). [28]
Cancer itself may impair OR. In young survivors of breast cancer with normal menstrual cycles, hormone and ultrasound measures of OR suggest decreased underlying OR when compared with their age-matched healthy women. [29] Chemotherapeutic drugs causes down regulation of FSH and LH levels. [30] Contrary to our study, Gunasegaram et al. reported an elevated concentration of LH in all stages of disease. [21]
AMH is a growth factor belongs to transforming growth factor-β family. In women, it is expressed by ovarian GC and controls the recruitment and growth of primary follicles and inhibition of FSH sensitivity of late antral follicles. As the pool of primordial follicles diminishes with aging, the follicle pool that secretes AMH also decreases. As a consequence, decreased serum AMH levels will increase the rate of recruitment of primordial follicles, resulting in an acceleration of the depletion of the primordial follicle pool. [31] Therefore, reduced AMH levels could be an excellent early marker for OR.
The results of the present study indicate a significant decrease in AMH levels in both pre-chemotherapy and post-chemotherapy group (group II and group III) when compared to control group (group I).
Su et al. and Jyoti et al. in their study on breast cancer patients, showed significantly lower AMH levels post-chemotherapy and diminished OR as compared to age-matched controls. [29,32] Anderson et al. observed that pre-treatment AMH concentrations were decreased with increasing age even before changes in other hormone concentrations occurred as compared to other ovarian tests, AMH seems to be the best marker reflecting the oocyte/ follicle pool. AMH levels showed a rapid and marked fall during the chemotherapy period, reaching undetectable concentrations in many women in their study. [33] Other studies had also reported that AMH levels dropped immediately after one and two cycles of chemotherapy, suggesting that the gonadotoxic effect of chemotherapy involves primary, secondary and early antral follicles. [34,35]
Thus, ovarian function can be challenging to assess, especially in the post chemotherapy setting. The potential markers of OR include biochemical markers and imaging assessments. [36,37]
No significant associations were seen between AMH and anthropometric, menstrual, or reproductive characteristics, including BMI, smoking status, menstrual cycle phase, and temporal or seasonal characteristics of blood collection. Changes in AMH appear to occur earlier than FSH and seem to reflect subtle changes in OR compared to FSH. Levels of AMH do not fluctuate significantly across the menstrual cycle, and levels are independent of FSH, LH, and E2 levels. This is mainly because AMH does not exert any feedback on the hypothalamic–pituitary–ovarian axis. This is in contrast to other ovarian hormones that display considerable cyclic variation. [31,38]
However, data on AMH values during the reproductive life span are limited, and it remains unknown whether AMH levels may enable age-independent prediction of an individual reproductive lifespan and spontaneous pregnancy in the general population. Unfortunately, the currently available measures to determine the postmenopausal status are of limited reliability. It is proposed that AMH can also be used to predict postmenopausal status in future studies.
Conclusion
Serum AMH levels were significantly decreased along with E2 and progesterone levels both before after chemotherapy in breast cancer patients along with nonsignificant alteration in levels of testosterone, FSH and LH. Hence AMH could be a valuable marker in these patients as it provides the exact knowledge of time by which the damage to granulosa cells and growing follicles occurs resulting in decreased ovarian reserve (OR); this is of great importance for the decision regarding the reproductive planning and optimal adjuvant hormonal treatment for women. Further studies are required to establish the use of these hormones as markers in appropriate stages of the disease before, after and up to at least one year after finishing adjuvant chemotherapy.
Conflict of Interest
All authors disclose that there was no conflict of interest.
REFERENCES
- Sariego J. Breast cancer in the young patient. The American surgeon. 2010; 76: 1397-1401.
- Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, et al. Breast cancer. In: Harrison’s Principles of Internal Medicine. Lippman ME (ed), 18th edn. New York: McGraw Hill. 2011; 754-763.
- Hernández L, Nuñez-Villarl MJ, Martínez-Arribas F, Pollán M, Schneider J. Circulating hormone levels in breast cancer patients. correlation with serum tumor markers and the clinical and biological features of the tumors. Anticancer Res. 2005; 25: 451-454.
- Su HI. Measuring ovarian function after cancer treatment. Menopausal medicine 2011; S1-S5.
- Oktem O, Oktay K. Quantitative assessment of the impact of chemotherapy on ovarian follicle reserve and stromal function. Cancer. 2007; 10: 2222-2229.
- Familiari G, Caggiati A, Nottola SA, Ermini M, Di Benedetto MR, Motta PM. Ultrastructure of human ovarian primordial follicles after combination chemotherapy for Hodgkin’s disease. Hum Reprod. 1993; 8: 2080-2087.
- Wulff C, Wilson H, Wiegand SJ, Rudge JS, Fraser HM. Prevention of thecal angiogenesis, antral follicular growth, and ovulation in the primate by treatment with vascular endothelial growth factor Trap R1R2. Endocrinology. 2002; 143: 2797-2807.
- Aubard Y, Piver P, Pech JC, Galinat S, Teissier MP. Ovarian tissue cryopreservation and gynecologic oncology: a review. Eur J Obstet Gynecol Reprod Biol. 2001; 97: 5-14.
- Partridge AH, Ruddy KJ, Gelber S, Schapira L, Abusief M, Meyer M, et al. Ovarian reserve in women who remain premenopausal after chemotherapy for early stage breast cancer. Fertil Steril. 2010; 94: 638-644.
- Henry NL, Xia R, Schott AF, McConnell D, Banerjee M, Hayes DF. Prediction of postchemotherapy ovarian function using markers of ovarian reserve. Oncologist. 2014; 19: 68-74.
- Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006; 24: 2917-2931.
- Lachgar M, Moulin G, Taieb J. A new immunoassay for estradiol on the Advia-Centaur (Siemens) system: analytical and clinical performances. Ann Biol Clin. 2012; 70: 581-589.
- Lee H, Park CJ, Lee G. Measurement of progesterone in human serum by isotope dilution liquid chromatography-tandem mass spectrometry and comparison with the commercial chemiluminescence immunoassay. Anal Bioanal Chem. 2010; 396: 1713-1719.
- Iamail AA, Cawood AM, Short F, Wakelin K, Wheeler M. Testosterone assay: guideline for the provision of a clinical biochemistry service. Ann Clin Biochem. 1986; 23: 135-145.
- Fanchin R, SchonaÈuer LM, Righini C, Guibourdenche J, Frydman RA, Taieb JE. Serum anti-MuÈ llerian hormone is more strongly related to ovarian follicular status than serum inhibin B, estradiol, FSH and LH on day 3. Hum Reprod. 2003; 18: 323-327.
- Wallace AM, Faye SA, Fleming R, Nelson SM. A multicentre evaluation of the new Beckman Coulter anti-Mullerian hormone immunoassay (AMH Gen II). Ann Clin Biochem 2011; 48: 370-373.
- Endogenous hormone and breast cancer collaborative group. Circulating sex hormones and breast cancer risk factors in post-menopausal women. Br J Cancer 2011; 105: 709-722.
- Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006; 354: 270-282.
- Christina S. Serum hormonal profile and its clinical utility in breast cancer patients among tamil women. J Adv Sci Re. 2013; 4: 24-26.
- Wiebe P. Progesterone metabolites in breast cancer. Endocrine-related cancer. 2006; 13: 717-738.
- Micheli A, Meneghini E, Secreto G, Berrino F, Venturelli E, Cavaerelli A, et al. Plasma testosterone and prognosis of postmenopausal breast cancer patients. J Clin Oncol. 2007; 25: 2685-2690.
- Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction. 2006; 132: 191-206.
- Fauser BC, Van Heusden A.M. Manipulation of human ovarian function: Physiological concepts and clinical consequences. Endocrine Reviews. 1997; 18: 71-106.
- Burger HG, Hale GE, Dennerstein L, Robertson DM. Cycle and hormone changes during perimenopause: the key role of ovarian function. Menopause. 2008; 15: 603-612.
- MacNaughton J, Banah M, McCloud P, Hee J, Burger H. Age related changes in follicle stimulating hormone, luteinizing hormone, oestradiol and immunoreactive inhibin in women of reproductive age. Clinical Endocrinology. 1992; 36: 339-345.
- Han HS, Ro J, Lee KS, Nam BH, Seo JA, Lee DH, et al. Analysis of chemotherapy-induced amenorrhea rates by three different anthracycline and taxane containing regimens for early breast cancer. Breast Cancer Research and Treatment. 2009; 115: 335-342.
- Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction. 2006; 132: 191-206.
- Pribylova O, Springer D, Svobodnik A, Kyr M, Zima T, Petruzelka L. Influence of chemotherapy to hormonal levels in postmenopausal breast cancer patients. Neoplasma. 2008; 55: 294-298.
- Su HI. Measuring ovarian function in young cancer survivors. Minerva Endocrinol. 2010; 35: 259-270.
- Falkson CI, Falkson HC, Falkson G. Effect of chemotherapy with or without buserelin on serum hormone levels in premenopausal women with breast cancer. Eur J Cancer Clin Oncol. 1991; 27: 1208-1211.
- Shaw CM, Stanczyk FZ, Egleston BL, Kahle LL, Spittle CS, Godwin AK, et al. Serum antimüllerian hormone in healthy premenopausal women. Fertil Steril. 2011; 95: 2718-2721.
- Bala J, Seth S, Dhanker R, Ghalaut VS. Chemotherapy: Impact on anti-müllerian hormone levels in breast carcinoma. JCDR. 2016; 10: BC19-BC21.
- Anderson RA, Themmen AP, Al-Qahtani A, Groome NP, Cameron DA. The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer. Human Reproduction. 2006; 21: 2583-2592.
- Lutchman SK, Muttukrishna S, Stein RC, McGarrigle HH, Patel A, Parikh B, et al. Predictors of ovarian reserve in young women with breast cancer. Br J Cancer. 2007; 96:1808-1816.
- Rosendahl M, Andersen CY, La Cour Freiesleben N, Juul Am Løssl K, Andersen AN. Dynamics and mechanisms of chemotherapy-induced ovarian follicular depletion in women of fertile age. Fertility and Sterility. 2010; 94: 156-166.
- Van Rooij IA, Tonkelaar ID, Broekmans FJ, Looman CW, Scheffer GJ, De Jong FH, et al. Anti-müllerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause. 2004; 11: 601-606.
- Van Rooij IA, Broekmans FJ, Te Velde ER, Fauser BC, Bancsi LF, De Jong FH, et al. Serum anti-Müllerian hormone levels: a novel measure of ovarian reserve. Hum Reprod. 2002; 17: 3065-3071.
- De Vos FYFL, Van Laarhoven HMW, Laven JSE, Themmend APN, Beex LVAM, Sweep CGJ, et al. Menopausal status and adjuvant hormonal therapy for breast cancer patients: A practical guideline. Critical Reviews in Oncology/Hematology. 2012; 84: 252-260.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.