Intramyometrial Vasopressin for Reducing Blood Loss at Myomectomy
Department of Obstetrics and Gynecology, Faculty of Clinical sciences, University of Abuja Teaching Hospital, PMB 228, Abuja, Nigeria
- *Corresponding Author:
- AD Isah
Department of Obstetrics and Gynecology
Faculty of Clinical sciences
University of Abuja Teaching Hospital
PMB 228, Abuja, Nigeria
Tel: 08061109664
E-mail: denisanthonyisah@yahoo.com
Citation: Isah AD, et al. Intramyometrial Vasopressin for Reducing Blood Loss at Myomectomy. Ann Med Health Sci Res. 2020;10: 857-864.
This is an open access article distributed under the terms of the Creative Commons Attribution‑NonCommercial‑ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non‑commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Background: Intra-myometrial injection of vasopressin during myomectomy is another method that could minimize of blood loss during myomectomy. However, there is paucity of knowledge to guide its use in myomectomy. Objective: To assess the efficacy of intra-myometrial vasopressin by determining whether it is noninferior to (as good as) a placebo in reducing intra-operative blood loss during abdominal myomectomy. Methods: This was a randomized, double-blind non-inferiority trial. This study was carried out in University of Abuja Teaching Hospital from September 2016 to May 2017. One hundred and twenty-eight patients with uterine leiomyoma of 14 – 30 weeks size they were randomized to either receive 20 IU (1 ml) of vasopressin in 19 mls of normal saline or 20 ml of normal saline at the planned uterine incision site during myomectomy after traditional tourniquet application. Myomectomy was then performed and the efficacy of vasopressin was assessed by a primary outcome and two secondary outcomes. P-value < 0.05 was considered statistically significant at 95% confident level. Results: A total of 128 women were randomized. However, only data from 125 women were analyzed. The mean estimated blood loss in the vasopressin group was 503.2 ml (SD, 97.5) compared with 841.3 ml (SD, 316.0) In the placebo group. The adjusted mean difference in intraoperative blood loss between the two groups, -359.3 (95% CI = -426.2 to -292.4), was statistically significant (p < 0.001). Post-operatively, the mean packed cell volume was significantly higher in the vasopressin group compared with the placebo group (29.5%, SD = 1.6 versus 27.7%, SD = 2.0; p<0.001). Furthermore, the need for blood transfusion was significantly higher in the placebo group compared with the vasopressin arm (OR =5.06; 95% CI = 2.07 – 12.4; p<0.001). Uterine size was a significant independent predictor of estimated blood loss in both groups (p < 0.001). Conclusion: Intra-myometrial vasopressin injection significantly reduced blood loss during abdominal myomectomy in this study.
Keywords
Vasopressin; Uterine fibriods; Myomectomy; Blood loss
Introduction
Uterine leiomyomas, commonly called fibroids are benign tumours of uterine myometrium composed of smooth muscle with variable amount of connective tissue. It is the commonest tumour of the female pelvic organ. [1] These common tumours are clinically apparent in 20% of women of reproductive age and may be present in up to 70% of uteri removed at hysterectomy. [2] Leiomyomas for unknown reasons occur 2-3 times more in black than in white women. [1,3] By their 5th decade, as many as 50% of black women will have leiomyomata. Although the precise cause of leiomyoma is unknown, advances have been made in the understanding of the hormonal, genetic, growth and molecular basis of these benign tumors. [4,5] Whether this higher prevalence among the blacks represents a genetic difference, or is perhaps associated with a higher cellular injury or inflammation resulting from an environmental agent remains unclear.[1,3] Uterine leiomyomas are also commoner in nulliparous and relatively infertile women. [6-8]
The standard treatment of symptomatic leiomyomas is hysterectomy for women who have completed childbearing and myomectomy for women who wish to preserve fertility. [9,10]
Myomectomy can be accomplished by laparotomy, laparoscopy, or hysteroscopy with laparotomy more commonly adopted in developing countries like Nigeria. [11-14] Massive blood loss associated with dissection of huge fibroids renders myomectomy a more technically challenging procedure than hysterectomy. [10,15] It is estimated that the average volume of blood loss during abdominal myomectomy ranges between 200 - 400 ml, [16-18] with blood loss greater than 1000 ml considered as major blood loss. [19] Surgical hemorrhage may result in hypovolemia and coagulation abnormalities. [20] A requirement for transfusion in up to 20% of cases following abdominal myomectomy has been reported in the literature, and in 2% of cases there was need for conversion of myomectomy to hysterectomy. [16,21,22]
A number of interventions have been introduced to reduce bleeding during myomectomy. These interventions include peri-cervical mechanical tourniquet application, the use of hormones such as GnRH analogues, vasopressin and uterotonics such as ergometrine, oxytocin, misoprostol and sulprostone. These have been used with varying degrees of success. [10] The advanced usage of myoma dissection techniques including the use of laser and chemical dissectors such as sodium-2- mercaptoethanesulfonate (mesna) is also reported in literature. [10,20] These later techniques of reducing blood loss are however, hardly available in most developing societies where it may be needed most. Despite the above-mentioned procedures, excessive hemorrhage during myomectomy remains a major challenge to gynecologic surgeons. [22]
Randomized trial data show that blood loss during myomectomy with vasopressin is significantly less than with placebo, [23-25] and less than or comparable to use of a uterine artery tourniquet. [26-28] The literature has also reported combining vasopressin with other measures. [25,29-32] There is paucity of data on the use of the vasopressin as a devise to reduce blood loss during abdominal myomectomy particularly in developing environments like Nigeria to the best of our knowledge. This study therefore, is designed to evaluate the efficacy of intra-myometrial injection of vasopressin compared to placebo in reducing blood loss at myomectomy.
Aim of the study
The aim of this study was to assess the efficacy of intramyometrial injection of vasopressin by determining whether it is noninferior to (as good as) placebo in reducing intra-operative blood loss during abdominal myomectomy.
Materials and Methods
This was a randomized, double-blind, active controlled, non-inferiority trial. It involves women presenting at the Gynaecologic clinic with leiomyomas during the study period and had given informed consent with uterine sizes of 14 - 30 weeks. Participants were randomized using block randomization to ensure a balance between the treatment arms. The participants picked out of a box to either receive 20 ml intra-myometrial injection of vasopressin (1 ml vasopressin diluted with 19 ml of normal saline) or 20 ml placebo (normal saline) at a planned incision site. Both groups had tourniquet applied at the base of the uterus close to the insertion of the uterosacral ligaments during the surgery. The drug or placebo was then injected along the pre- planned incision site on the uterus directly into the myometrium. Before injecting the drug or placebo, the plunger was withdrawn to ensure that the syringe was not in a blood vessel and then myomectomy was then performed.
At every step, precautions were taken to reduce blood loss during the procedure. The tourniquet was released intermittently after every 30 minutes to avoid tissue necrosis. After removal of the myoma, dead spaces were obliterated using Vicryl 2 sutures. The serosa was closed with nylon 2/0 in a baseball pattern and the tourniquet removed. Where there was bleeding after the release of the tourniquet, this was taken care of by applying sutures. The efficacy of vasopressin was measured by comparing amount of blood loss during surgery, change in the haematocrit, and need for blood transfusion postoperatively in the treatment and control groups. Estimation of blood loss was done by measuring the amount of blood in the suction bottle and by counting the mops used during the operation. Mops were weighed before and after the procedure. The total blood loss was calculated by adding blood in the suction bottle and the amount gotten from the mops used (postoperative weight of mops minus preoperative weight of mops).
The primary outcome was the estimated intra-operative blood loss during abdominal myomectomy. Estimated blood loss was considered major if it was greater than 1000 ml. A secondary outcome, need for blood transfusion, was assessed two days postoperatively and compared between the treatment and control groups.
Statistical analysis
Baseline characteristics of participants in the two treatment groups were reported using frequency distributions and descriptive statistics including measures of central tendency and dispersion. The preliminary analysis of our primary outcome measure was a z- test comparing the mean blood loss in each treatment group. Further, linear regression analysis examined the effect of adjustment for relevant covariates which could be predictors of the outcome or that may be imbalanced at the baseline.
The primary outcome, mean blood loss, was assessed for correlation with the secondary outcomes, need for blood transfusion and postoperative haematocrit. The study outcome measures were analysed primarily per protocol, and therefore included only participants who completed the trial as originally allocated. For sensitivity reason, analyses were repeated using the intent to treat approach. The results of both approaches should support noninferiority, if not the results would be inconclusive. For the primary efficacy outcome, estimated blood loss, lower values represent better result; thus, noninferiority of vasopressin to tourniquet will be inferred if the lower limit of the CI (for the difference in the mean amount of blood loss intraoperatively during myomectomy) falls below the noninferiority margin. Data was fed into and analysed IBM Statistical Package for Social Sciences (SPSS) package version 20.0, August 2016 (SPSS 20.0, IBM, Armonk, NY, United States of America).
The analyses in this study were two-tailed and P-value < 0.05 was considered statistically significant at 95% confident level.
Results
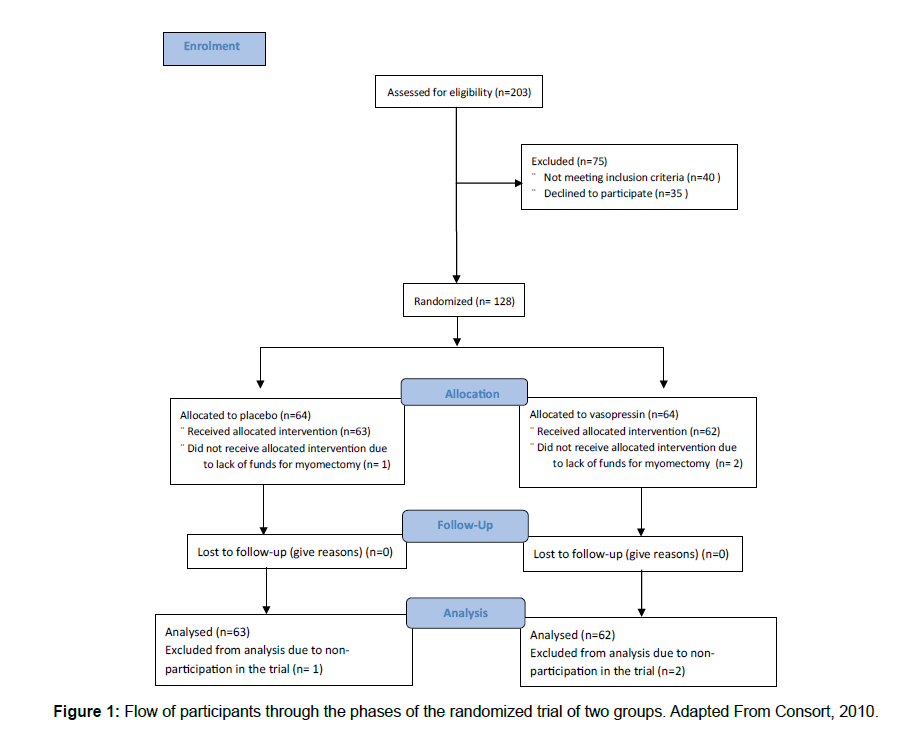
A total of 203 women were screened for eligibility, 128 met the eligibility criteria and were randomized between September 2016 and May 2017. One Hundred and twenty-five women completed the trial, while 3 women dropped out citing inability to pay for the surgery [Figure 1].
Baseline demographic and clinical characteristics are shown for both groups in Table 1. The average age of participants in the study sample was 33.4 (SD, 3.8) years, and ranged from 27 to 43 years. Over 65% of the study participants were single, while 77% of participants in placebo group had a parity of zero compared to 64% in the vasopressin group. The mean uterine size among participants in the vasopressin group was 21.8 (SD, 3.7) weeks, compared to 21.0 (SD, 4.2) in the placebo group. In both groups, the average preoperative PCV was 32.1 (SD, 1.4). Over 70% of the myomectomy was performed by consultant surgeons in both groups, and general anesthesia was used in over 50% of myomectomy in both groups. There was no statistically significant difference between placebo and vasopressin group in the baseline characteristics [Table 1].
| Baseline Characteristics | Placebo Group (n=63) |
Vasopressin Group (n=62) |
Total n (125) |
p-value |
|---|---|---|---|---|
| Age, mean (SD), years | 32.8 (3.4) | 33.9 (4.1) | 33.4 (3.8) | 0.11 |
| Marital Status Single Married |
46 (71.9) 18 (28.1) |
41 (64.1) 23 (35.9) |
87 (68.0) 41 (32.0) |
0.34 |
| Parity 0 1-2 >=3 |
49 (76.6) 11 (17.2) 4 (6.3) |
41 (64.1) 13 (20.3) 10 (15.6) |
90 (70.3) 24 (18.8) 14 (10.9) |
0.18 |
| Uterine size, mean (SD), weeks | 21.0 (4.2) | 21.8 (3.7) | 21.4 (4.0) | 0.25 |
| Pre-operative PCV, mean (SD) | 32.1 (1.4) | 32.1 (1.4) | 32.1 (1.4) | 0.80 |
| Cadre of Surgeon Consultants Registrars |
47 (74.6) 16 (25.4) |
44 (71.0) 18 (29.0) |
91 (71.1) 34 (26.6) |
0.65 |
| Anaesthesia General Spinal |
44 (69.8) 19 (30.2) |
46 (74.2) 16 (25.8) |
90 (72.0) 35 (28.0) |
0.59 |
| Intra- and Post-Operative Characteristics | ||||
| Blood Loss (%) <500 500 ? 1000 >1000 |
2 (3.2) 51 (81.0) 10 (15.9) |
19 (30.7) 43 (69.4) 0 (0) |
21 (16.8) 94 (75.2) 10 (8.0) |
<0.001 |
| Postoperative PCV <30 >30 |
51 (81.0) 12 (19.1) |
30 (48.4) 32 (51.6) |
81 (64.8) 44 (35.2) |
<0.001 |
| Change between pre- and post-operative PCV, mean (SD) | 4.44 (1.39) | 2.66 (0.75) | 3.56 (1.43) | <0.001 |
| Number of blood units transfused 0 1 2 ? 4 |
36 (57.1) 5 (7.9) 22 (34.9) |
54 (87.1) 8 (12.9) 0 (0) |
90 (72.0) 13 (10.4) 22 (17.6) |
<0.001 |
Table 1: Characteristicsa of study sample by placebo and treatment group.
Major blood loss >1000 ml was recorded only among women in the placebo group. A greater proportion of women in the vasopressin group (52%) had PCV greater than 30% compared with 19% in the placebo group. The maximum number of blood units transfused in the placebo group was 4 units, compared to 1 unit in the vasopressin group. About 35% of women in the placebo group received between 2 – 4 units of blood, while no woman in the vasopressin group required more than 1 unit of blood. For the intra- and post-operative characteristics, placebo and vasopressin groups differed significantly [Table 1].
Blood loss from myomectomy
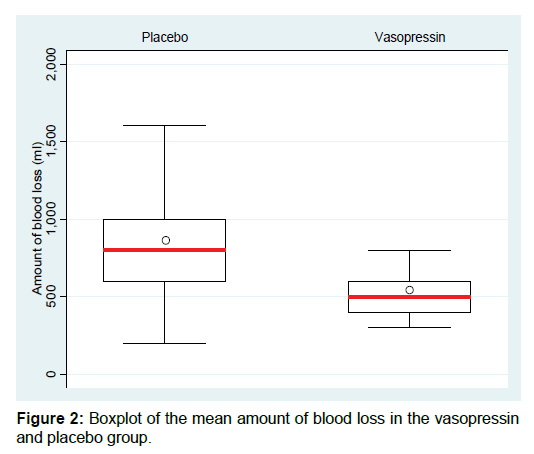
The mean amount of blood loss in the vasopressin group was 503.2 (SD, 97.5, median = 500, range = 300 – 800ml) ml compared with 841.3 (SD, 316.0, median = 800, range = 200 – 1800ml) ml in placebo group. The distribution for the placebo group shows a wider spread around its mean, hence higher variability compared to the vasopressin group whose distribution is more compact [Figure 2]. Furthermore, there were 10 cases of excessive blood loss (>1000ml), all of which were in the placebo group. Also, the mean and median blood loss was lower in the vasopressin group than the placebo group. A z-test was conducted to determine if the difference in mean estimated blood loss between the placebo and vasopressin groups was statistically significant. This test showed that the difference of -338.04 ml (95% CI = -256.3 – -419.8) in mean estimated blood loss between the placebo and vasopressin groups was statistically significant, with a p-value of <0.001 [Table 2]. Estimated blood loss was significantly decreased by 40% in vasopressin group compared with the placebo group.
| Variables | Placebo | Vasopressin | Difference/Odds ratio | p-value |
|---|---|---|---|---|
| Blood loss, mean (SD) ml | 841.3 (316.0) | 503.2 (97.5) | -338.04 (-421.1 ? -255.0) | <0.001 |
| Postoperative PCV, mean (SD) ml | 27.7 (2.0) | 29.5 (1.6) | 1.78 (1.15 ? 2.42) | <0.001 |
| Need for blood transfusion (%) No Yes |
36 (57.1) 27 (42.9) |
54 (87.1) 8 (12.9) |
5.06 (2.07 ? 12.4) | <0.001 |
Table 2: Bivariate analysis of the primary and secondary outcome measures.
Packed cell volume change and post-operative packed cell volume
The mean change between pre- and post-operative PCV was higher in the placebo group (mean=4.4, SD=1.39, range= 1 – 9) compared with vasopressin group (mean=2.7, SD=0.75, range=2 – 5). The difference in PCV change between both groups (1.78; 95% CI= 1.39 – 2.17) is statistically significant (p<0.001), as assessed by the z-test. This result shows that entire sample experienced a drop in PCV between pre- and postmyomectomy, however, the reduction in PCV was greater in the placebo group compared to vasopressin group.
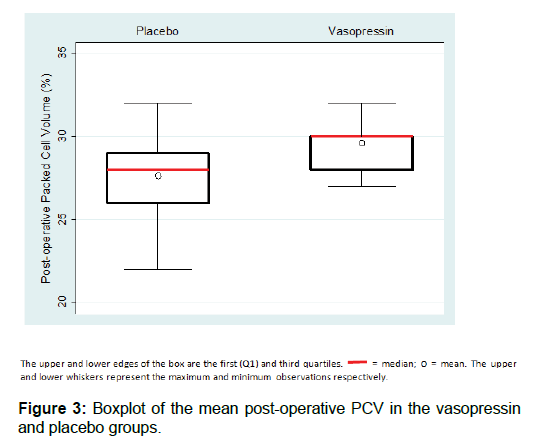
The, postoperative PCV was higher among the vasopressin group 29.5% (SD = 1.6, median = 30, range = 29.1 – 29.8), compared with the placebo group, 27.7% (SD = 2.0, median = 28, range = 27.2 – 28.2). The post-operative PCV was higher in the vasopressin group compared with the placebo group [Figure 3]. The difference in mean post-operative PCV between vasopressin and placebo group, 1.78 (95% CI = 1.15 – 2.42) was statistically significant (p-value <0.001), as assessed using a z-test [Table 2].
Need for blood transfusion
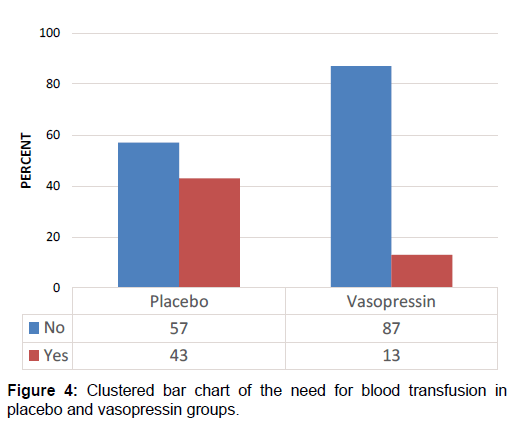
Among participants in the vasopressin group, 8 (13%) needed blood transfusion post-operatively compared to 27 (43%) in the placebo group [Figure 4]. An odds ratio analysis showed that the odds ratio of the need for blood transfusion in placebo versus vasopressin group was 5.06 (95% CI = 2.07 – 12.4). This means that women who received placebo during abdominal myomectomy had 5.06 times higher odds of needing blood transfusion compared to their counterparts who received vasopressin. Given that the odds ratio was greater than 1, it means that women in the placebo group were more likely to need blood transfusion postoperatively than the vasopressin group [Table 2].
Linear regression of blood loss on treatment group and other covariates
The regression slope coefficient for vasopressin group is -359.6 (-427.6 – -291.5). This means that on average, blood loss decreased by 359.6 ml during abdominal myomectomy for women in the vasopressin group compared to women in the placebo group, all other independent variables being held constant. Except for uterine size, the associations between blood loss at myomectomy and the other independent variables were not statistically significant [Table 3]. There was no adverse effect of vasopressin observed in either group.
| Variables | Adjusted Coefficient (95% CI) | p-value |
|---|---|---|
| Treatment group Placebo Vasopressin |
0 -359.3 (-426.2 ? -292.4) |
<0.001 |
| Uterine size | 41.8 (31.5 ? 52.1) | <0.001 |
| Cadre of surgeon Consultant Registrar |
0 16.6 (-57.9 ? 91.0) |
0.66 |
| Type of anaesthesia General Spinal |
0 -19.2 (-93.5 ?55.0) |
0.61 |
| Age | -11.5 (-22.2 ? 0.68) | 0.04 |
Table 3: Multivariate linear regression analyses of blood loss on treatment group and other co-variates.
Discussion
This randomized controlled trial assessed the efficacy of intramyometrial vasopressin for reducing intraoperative blood loss during abdominal myomectomy. Among women undergoing abdominal myomectomy, intra-myometrial vasopressin injection reduced the amount of blood loss intraoperatively, compared to placebo. Also, postoperative packed cell volume was higher, and the need for blood transfusion was lower, 2 days post operatively among women that received intramyometrial vasopressin, compared to their counterparts that received placebo.
Myomas are diagnosed in 20-25% of women of reproductive age and 30-40% of women older than 40 years. [33] It was reflected in this study in which the women fell within the reproductive age group. Uterine fibroids are commoner in nulliparous and relatively infertile women than the parous and fertile ones. [6] This was seen in this study as 70.3% of the women were nulliparous.
The efficacy of vasopressin demonstrated in this study concurred with the results of previous randomized controlled trials that compared vasopressin to placebo or nothing, showing that blood loss was less with vasopressin than with placebo. [10,23,29] The use of vasopressin resulted in less intraoperative blood loss, 503.2 ml (SD, 97.5, median = 500) in the vasopressin group compared with 841.3 ml (SD, 316.0, median = 800) in the placebo group. Acting through V1 receptors in the myometrium, vasopressin causes vasoconstriction, thus reducing the amount of blood that is lost. [29,34]
However, the amount of blood loss in both groups in the present study is higher compared to other studies. For example, one study reported a median blood loss of 225 ml (range 150-400 ml) in the vasopressin group compared with 675 ml (range 500- 800 ml) in placebo group, [23] while in another study, the median blood loss in vasopressin group was 210 ml (range 70 – 1020 ml) and in the placebo group 500 ml (45 – 2850 ml). [29] The difference in blood loss between the present study and other studies may be due to how the trial was conducted; in one of the studies, there were 20 participants, and the trial was stopped halfway due to heavy bleeding in 10 of the study participants. In the other study, participants were not randomised, but were picked at the discretion of the surgeon. The variability could also be due to variable methods of administration of the vasopressin; in one study, vasopressin was not injected at the planned incision site, but in the broad ligament. In another study, although retrospective, women who received pitressin and/or tourniquet had lesser intraoperative estimated blood loss compared with women who received no haemostatic treatment.
Overall, results in the present study follow the trend observed in the literature that vasopressin reduces blood loss during myomectomy (abdominal and laparoscopic), when compared with placebo or no treatment.
The mean postoperative hematocrit was higher in the vasopressin group compared to the placebo group. Also, the decrease in PCV was significantly higher for the placebo group than the vasopressin group. These results concur with findings from other studies which showed that decrease in hematocrit was higher for participants in placebo group compared to those in vasopressin group. [23,29]
Forty-three percent (43%) of the placebo group needed blood transfusions compared with thirteen percent (13%) in the vasopressin group. Study participants who did not receive vasopressin during myomectomy had a significantly higher chance of being transfused than who received vasopressin. This finding is comparable with findings from other studies. In a systematic review of interventions to reduce haemorrhage during myomectomy for fibroids, [10] the need for blood transfusion was found to be significantly reduced in vasopressin compared to placebo group. [23] However, in a retrospective study of perioperative morbidities from abdominal myomectomy in 128 women, LaMorte et al. found a higher rate of blood transfusion (28%) in women who received pitressin and/or vasopressin than in women who did not receive any prophylactic hemostatic measures. [22] This difference could be due to various reasons. The prophylactic hemostatic measures were taken only in 44 women, and these women may have had poorer preoperative PCV, or larger myomas. Also, it was not clear how many of the 44 patients did receive the medication. Furthermore, the authors of that study evaluated the necessity of blood transfusion based on certain criteria and found that in 8 cases transfusion was unnecessary; thus, some of the transfusions among these 44 women may have been unnecessary.
Regarding postoperative PCV and the need for blood transfusion, the observed result is to be expected as blood loss correlated well with postoperative PCV and the need for blood transfusion. [16]
Worthy of note is the fact that all participants in this study had tourniquet application, as it would not be ethical to conduct myomectomy without any blood loss-reducing treatment when there exists a proven, effective, standard treatment in use in this setting. Also, myomectomy without uterine vessel occlusion results in so much hemorrhage that is not recommended for uterine sizes more than 12 weeks. [27] Although not the goal of this trial, using a combination of vasopressin plus tourniquet was more efficacious in terms of all the outcomes studied. This finding is in line with other studies where vasopressin was used in combination with tourniquet compared to vasopressin alone, tourniquet alone. The blood loss from the group that had vasopressin and tourniquet was lower compared to the group that had vasopressin alone or tourniquet alone. [22,25] A retrospective that compared between study also found lower blood loss when vasopressin was combined with tourniquet compared with vasopressin alone. However, there was no significant difference between groups regarding change in hematocrit, and the need for blood transfusion. [32]
In this study also, blood loss was found to be correlated to the uterine size even after adjusting for other factors. This was also seen in a study where subjects were stratified by ultrasounddetermined uterine volume into <600 cm3 and >600 cm3 and then randomised into treatment groups. The conclusion was that blood loss during myomectomy is primarily incurred while operating on the uterus and is correlated with preoperative uterine size, total weight of fibroids removed, and operating time. [26] Similarly, Iverson et al. found in their retrospective cohort study that intraoperative blood loss was primarily related to uterine size and not the procedure. [16]
However, the cadre of surgeon and anesthetic techniques had no effect on blood loss in this study. This could be explained by the fact that, most of the operations were carried out by the consultants and general anesthesia was used in most of the operations. There are reports of severe complications when vasopressin was inadvertently injected intravenously during local infiltration. In this study, great care was always taken not to inject the solution intravascular. [35,36]
Although we only compared between vasopressin and placebo, studies that have compared between vasopressin and other agents have found lower blood loss, smaller decrease in PCV, higher post-operative PCV and reduced need for blood transfusion with the use of vasopressin during abdominal myomectomy. This implies that vasopressin is quite efficacious in reducing blood loss during abdominal myomectomy. [27,28]
Conclusion
The results indicate intra-myometrial vasopressin injection is efficacious in reducing blood. Thus, it can be concluded that intra-myometrial vasopressin injection is noninferior to placebo (normal saline) in reducing blood loss during abdominal myomectomy. Since the present study has shown that vasopressin is not inferior to placebo, vasopressin may be considered for use in reducing intraoperative blood loss during abdominal myomectomy. However, in our setting, tourniquet is the hemostatic intervention of choice, thus further studies may be needed to assess whether vasopressin is superior to conventional tourniquet application before it can be adopted.
Competing Interests
The authors declare that they have no competing interests.
References
- Marshall LM, Spiegelman D, Barbieri RL, Goldman MB, Manson JE, Colditz GA, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973.
- Lumsden M. Uterine Leiomyomata. In: Edmonds D, editor. Dewhurst’s Textbook of Obstetrics and Gynaecology for postgraduates. 7th ed. London: Blackwell Science; 2007. p. 638-644.
- Drinville J, Mermarzadeh S. Leiomyoma of the Uterus. In: Current diagnosis and treatment Obstetrics and Gynaecology. 10th ed. New York: Mc Graw Hill, USA, 2007; 639-653.
- Parker H. Uterine myomas: management. Fertil Steril. 2007;87:725-736.
- Wallach EE, Buttram VC, Reiter RC. Uterine leiomyomata: etiology, symptomatology, and management. Fertil Steril. 1981;36:433-445.
- Ogunniyi S, Fasubaa O. Uterine fibromyomata in Ilesha, Nigeria. Niger Med Pr. 1990;19:93-95.
- Day Baird D, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: Ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Emembolu JO. Uterine fibromyomata: Presentation and management in Northern Nigeria. Int J Gynecol Obstet. 1987;25:413-416.
- Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016;22:665-686.
- Kongnyuy EJ, Wiysonge CS. Interventions to reduce haemorrhage during myomectomy for fibroids. Cochrane Database Syst Rev. 2014.
- Okezie O, Ezegwui HU. Management of uterine fibroids in Enugu, Nigeria. J Obstet Gynaecol (Lahore). 2006;26:363-365.
- Ikechebelu JI, Ezeama CO, Obiechina NJA. The use of torniquet to reduce blood loss at myomectomy. Niger J Clin Pract. 2010;13:154-158.
- Ezeama C, Ikechebelu J, Obiechina N, Ezeama N. Clinical Presentation of Uterine Fibroids in Nnewi, Nigeria: A 5-year Review. Ann Med Health Sci Res. 2012;2:114-118.
- Adesina KT, Owolabi BO, Raji HO, Olarinoye AO. Abdominal myomectomy: A retrospective review of determinants and outcomes of complications at the University of Ilorin Teaching Hospital, Ilorin, Nigeria. Malawi Med J. 2017;29:37-42.
- Lethaby A, Puscasiu L, Vollenhoven B. Preoperative medical therapy before surgery for uterine fibroids. Cochrane Database Syst Rev. 2017.
- Iverson RJ, Chelmow D, Strohbehn K, Waldman L, Evantash E. Relative morbidity of abdominal hysterectomy and myomectomy for management of uterine leiomyomas. Obstet Gynecol. 1996;88:415-419.
- West S, Ruiz R, Parker WH. Abdominal myomectomy in women with very large uterine size. Fertil Steril. 2006;85:36-39.
- Sawin SW, Pilevsky ND, Berlin JA, Barnhart KT. Comparability of perioperative morbidity between abdominal myomectomy and hysterectomy for women with uterine leiomyomas. Am J Obstet Gynecol. 2000;183:1448-1455.
- Harris WJ. Early complications of abdominal and vaginal hysterectomy. Obstet Gynecol Surv. 1995;50:795-805.
- Parker H. Techniques to reduce blood loss during abdominal or laparoscopic myomectomy - UpToDate. Sharp T, Falcone T, Falk J, editors. UpToDate. Waltham, MA: UpToDate Inc.;
- Aubuchon M, B Pinto A, B Williams D. Treatment of uterine fibroids. Vol. 9, Primary Care Update for OB/GYNS. 2002;231-237.
- LaMorte AI, Lalwani S, Diamond MP. Morbidity associated with abdominal myomectomy. Obstet Gynecol. 1993;82:897-900.
- Frederick J, Fletcher H, Simeon D, Mullings A, Hardie M. Intramyometrial vasopressin as a haemostatic agent during myomectomy. Br J Obstet Gynaecol. 1994;101:435-437.
- Wong ASW, Cheung CW, Yeung SW, Fan HL, Leung TY, Sahota DS. Transcervical Intralesional Vasopressin Injection Compared With Placebo in Hysteroscopic Myomectomy. Obstet Gynecol. 2014;124:897-903.
- Zhao F, Jiao Y, Guo Z, Hou R, Wang M. Evaluation of loop ligation of larger myoma pseudocapsule combined with vasopressin on laparoscopic myomectomy. Fertil Steril. 2011;95:762-726.
- Ginsburg ES, Benson CB, Garfield JM, Gleason RE, Friedman AJ. The effect of operative technique and uterine size on blood loss during myomectomy: a prospective randomized study*†*Supported in part by CLINFO grant RM01RR02635 from the National Institutes of Health, Bethesda, Maryland. †Presented at the 48th Annual Mee. Fertil Steril. 1993;60:956-962.
- Fletcher H, Frederick J, Hardie M, Simeon D. A randomized comparison of vasopressin and tourniquet as hemostatic agents during myomectomy. Obstet Gynecol. 1996;87:1014-1018.
- Saha MM, Khushboo, Biswas SC, Alam H, Kamilya GS, Mukhopadhyay M, et al. Assessment of blood loss in abdominal myomectomy by intramyometrial vasopressin administration versus conventional tourniquet application. J Clin Diagnostic Res. 2016;10:QC10-QC13.
- Kimura T, Kusui C, Matsumura Y, Ogita K, Isaka S, Nakajima A, et al. Effectiveness of hormonal tourniquet by vasopressin during myomectomy through vasopressin V1a receptor ubiquitously expressed in myometrium. Gynecol Obstet Invest. 2002;54:125-131.
- Frederick S, Frederick J, Fletcher H, Reid M, Hardie M, Gardner W. A trial comparing the use of rectal misoprostol plus perivascular vasopressin with perivascular vasopressin alone to decrease myometrial bleeding at the time of abdominal myomectomy. Fertil Steril. 2013;100:1044-1049.
- Ngichabe S, Obura T, Stones W. Intravenous tranexamic acid as an adjunct haemostat to ornipressin during open myomectomy. A randomized double blind placebo controlled trial. Ann Surg Innov Res. 2015;9:10.
- Kathiresan ASQ, Abdelfattah SR, Romary LM, Gonzalez-Quintero VH, Verma U. Vasopressin and tourniquets: a comparison of blood loss in patients undergoing abdominal myomectomies. Fertil Steril. 2008;90:S458.
- Stewart E, Cookson C, Gandolfo R, Schulze-Rath R. Epidemiology of uterine fibroids: A systematic review. BJOG An Int J Obstet Gynaecol. 2017;124:1501-1512.
- Liedman R, Grant L, Igidbashian S, James I, McLeod A, Skillern L, et al. Intrauterine pressure, ischemia markers, and experienced pain during administration of a vasopressin V1a receptor antagonist in spontaneous and vasopressin-induced dysmenorrhea. Acta Obstet Gynecol Scand. 2006;85:207-211.
- Barrett KE, Barman SM, Boitano S, Brooks H. Ganong’s Review of Medical Physiology. 24th ed. Mcgraw-Hill, USA; 2012;768
- Riess ML, Ulrichs JG, Pagel PS, Woehlck HJ. Severe Vasospasm Mimics Hypotension After High-Dose Intrauterine Vasopressin. Anesth Analg. 2011;113:1103-1105.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.