Investigation the Development of Metabolic Syndrome for Type 2 Female Diabetic through Serpin E1, Chemoattractant Protein-1 and Adiponectin
Received: 16-Oct-2022, Manuscript No. AMHSR-22-77440; Editor assigned: 18-Oct-2022, Pre QC No. AMHSR-22-77440 (PQ); Reviewed: 01-Nov-2022 QC No. AMHSR-22-77440; Revised: 22-Feb-2023, Manuscript No. AMHSR-22-77440 (R); Published: 01-Mar-2023, DOI: 10.54608.annalsmedical.2023.91
Citation: Kinani BHA, et al. Investigation the Development of Metabolic Syndrome for type 2 Femal Diabetic through Serpin E1, Chemoattractant Protein-1 and Adiponectin. Ann Med Health Sci Res. 2023;13:482-491
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
In this study, 54 female patients with type 2 diabetes and 36 non-diabetics took part, with obese and non-obesity persons compared to an apparently healthy control group. The serpin E1 and monocyte chemoattractant protein-1 were measured by ELISA sandwich while the colorimetric methods were used for calculating HbA1C, serpin E1 (serine proteinase inhibitors) and lipid peroxidation. The levels of serpin E1 (PAI-1) and monocyte chemo attractant protein -1 in the blood of non-obese women differed signi icantly from those of obese individuals (p=0, 05). Similarly, there was a signi icant difference between the receptor levels of no obese female patients and serum controls from obese females (p=0.05). The values of serpin E1, monocyte chemoattractant protein -1, HbA1C were increased in patients with type 2 diabetes who have the highest TG, TC, vLDL, LDL and also decreased for HDL and adiponectin in obese females. In this study, parameter was compared with adiponectin under review.
Keywords
Type 2 diabetes mellitus; Obesity; Serpin E1; Monocyte Chemoattractant Protein-1(MCP); HbA1C; Lipid peroxidation
Introduction
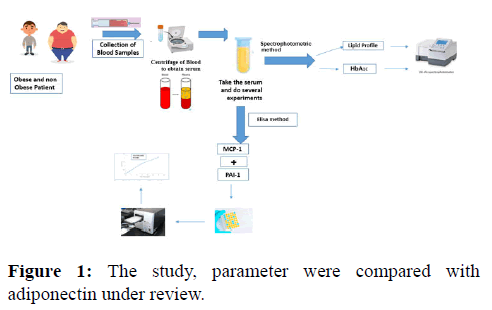
Research into the role of the C-C chemokine Monocyte Chemoattractant Protein 1 (MCP-1) plays in the development of metabolic syndrome and type 2 diabetes has skyrocketed during the past two decades. The purpose of this article is to present a synopsis of this chemokine, including its development, regulatory mechanisms, functions, and therapeutic approaches. This research emphasizes the role of MCP-1 in the onset of obesity, diabetes, cardiovascular disease, insulitis, diabetic nephropathy, and diabetic retinopathy. In white adipose tissue, simultaneous ablation of AdipoR1 and R2 resulted in elevated MCP-1 expression and the macrophage marker MAC-1 (WAT). Reduced adiponectin signaling could be an upstream mechanism for increased MCP-1 production and inflammation in WAT. Obesity, a chronic condition characterized by adipose tissue growth and inflammatory components, has been associated to the development of a number of metabolic illnesses. Insulin resistance and metabolic syndrome are more frequent in people who have abdominal obesity and excess Visceral Adipose Tissue (VAT). VAT plays a critical role in obese inflammation. These abnormalities increase before the development of T2D and are strongly linked to NAFLD (Nonalcoholic Fatty Liver Disease). Obesity, Cardio Vascular Disease (CVD), and atherosclerosis are all linked to high cholesterol levels. Chemokines mediate immunlogical responses to these circumstances, resulting to an innate immune response characterized by the generation of cytokines. HbA1c measures eight to twelve weeks of mean plasma glucose. It may be done anytime and does not need fasting. Due to its features, it's the key test for glycemic control in diabetics. Newly, there has been a lot of interest in employing it as a diabetes analytical test and a diabetes showing test for high risk persons. The danger of developing prediabetes and type 2 diabetes over a 10 years follow-up period in a community based sample was similarly found to be associated with higher PAI-1 and lower adiponectin levels. Recent research has connected PAI-1 to hyperlipidemia and revealed a role for PAI-1 in lipid metabolism. Further, pharmaceutical PAI-1 inhibition protects against hepatic steatosis, dyslipidemia, and reduces total chop. Increased adipose tissue in obese people causes hypoxia and the initiation of inflammasomes, which in turn stimulates the synthesis of adipocytes and the initiation of monocytes, resultant in chronic low grade inflammation. In addition, low grade inflammation contributes to the development of atherosclerosis via processes such as changes in monocyte activity. In individuals with metabolic illnesses like T2D, monocytes production a crucial role in initiating endothelial and vascular dysfunction, which are major predictors of thrombosis and death. As a result, chronic immunological activation is being addressed in an attempt to decrease CVD risk in people with metabolic illnesses by inhibiting the production of cellular mediators of inflammation and thrombosis, such as monocytes. MCP-1 activation may cause type 2 diabetes in obese patients. Increased adhesion molecules and chemokines in the endothelium may accompany pathogenic changes, activating and attracting circulating monocytes into sub endothelial space where they develop into macrophages [1-5] (Figure 1).
This process promotes atherosclerosis and subsequent CVDs. Fructose ingestion exacerbated the already elevated levels of MCP-1 in morbidly obese people. To add insult to injury, MCP-1 signaling has a direct function in the expansion of obesity. Due to its antigenic action on endothelial cells, MCP-1 may aid in the development and reshaping of fat tissues. Obesity related disorders, including insulin resistance and type 2 diabetes, can be improved by reducing MCP-1 overproduction, which must be done reduced proportion converses activity is associated with increased cholesterol levels [6,7].
Materials and Methods
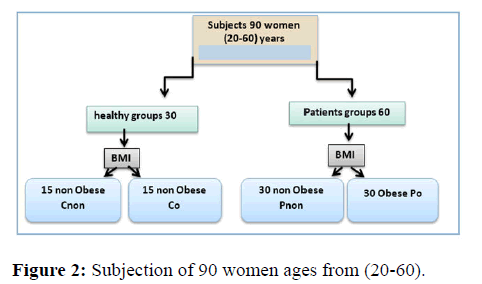
Method: In this study, participants were separated into two groups: diabetics and non-diabetics, the patients were separated into two groups (obese and non-obese). Also the control groups divided into two matching with patients group, in the study divided into two groups (Figure 2).
The investigation was carried out in the endocrinology center of Al-Murjan Al-Talafi Hospital in Al-Hilla, Babylon, Iraq, for a case control study. The following are patient and healthy person lists: type 2 diabetes patients (ages 26 to 60) who came to the diabetes research center Individuals and sick were both subjected to interrogators and legislation. The investigation was completed. To collect, use parameters such as pathological sex and. Background and weights According to the guidelines, (BMI, kg/m2) was calculated as average weight (23.11-26.69) (kg/m2) or obesity (>30 kg/m2).
Blood sampling
Patients and observers had 5 ml blood taken. Before being centrifuged at 4500 rpm for 10 minutes, blood samples might clot. The path was split and buried. Hold till analysis is done.
Statistical analysis
The data was analyzed using SPSS Statistics. The findings were analyzed using means, standard deviations, and ANOVA to determine the difference between groups. Students' assessments were compared to two average semesters. Pearson's correlation coefficients were used to compare the relationships between variables. P-values the test had two ends, and a P-value of 0.05 was thought to be significant.
Results and Discussion
Clinical properties
Biochemical and clinical factors experienced by the study's participants are listed in Tables 1 and 2. Table 1 shows the clinical characteristics of DM2 patients and healthy controls. The age difference between the two groups was not statistically significant (p>0.05), and while FBG levels were higher in the patients group than in the controls group, the BMI difference was not statistically significant (p>0.05).
| Parameters | Patients | Control | P-value |
|---|---|---|---|
| Age (years) | 48.80 ± 13.13 | 52.48 ± 13.41 | 0.011 |
| BMI (kg/m2) | 29.46 ± 5.05 | 27.53 ± 6.05 | 0.149 |
| HbA1C | 9.34 ± 1.27 | 5.17 ± 0.79 | 0.00001 |
| T.C (mg/dL) | 266.71 ± 62.13 | 130.07 ± 30.08 | 0.00001 |
| T.G (mg/dl) | 253.52 ± 100.64 | 118.35 ± 23.66 | 0.00001 |
| HDL-C (mg/dL) | 44.23 ± 7.13 | 50.98 ± 9.16 | 0.00001 |
| LDL (mg/dL) | 171.77 ± 55.94 | 55.41 ± 32.81 | 0.00001 |
| VLDL (mg/dL) | 50.70 ± 20.12 | 23.67 ± 4.73 | 0.00001 |
| Duration of disease (years) | 5.39 ± 2.69 | - | |
| Duration of medication (years) | 4.83 ± 2.17 | - | |
| MCP-1 | 214.60 ± 141.91 | 85.58 ± 15.10 | 0.00001 |
| PAI-1 | 5.14 ± 2.53 | 2.11 ± 1.55 | 0.00001 |
| The mean difference is significant at p ≤ 0.05. | |||
Table 1: Clinical properties for DM2 patients and controls.
Measurement of HbA1C and BMI
Table 2 shows the results of a comparison of HbA1C and body mass index between patient and control groups. Obese patients have high HbA1C (9.44 ± 1.38), with BMI (33.543.31 ±) compared to non-obese patients HbA1C (9.23 ± 1.16), with BMI (25.37 ± 2.53), according to criteria compared to obese control HbA1C (4.93 ± 0.75), with BMI (32.97 ± 2.99) compared another non obese control HbA1C (5.71 ± 0.78), with BMI (22.42 ± 2.82).
| Parameter | N | Groups | Mean ± SD | CI 95% | Compared groups | P value | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| BMI | 30 | Po | 3.31 ± 33.54 | 32.3 | 34.78 | Po | Pnon | 0.00001 |
| Co | 0.543 | |||||||
| Cnon | 0.00001 | |||||||
| 30 | Pnon | 2.53 ± 25.37 | 24.42 | 26.32 | Pnon | Co | 0.00001 | |
| Cnon | 0.002 | |||||||
| 15 | Co | 2.99 ± 32.9 7 | 31.32 | 34.63 | Co | Cnon | 0.00001 | |
| 15 | Cnon | 2.82 ± 22.42 | 20.85 | 23.98 | ||||
| HbA1C | 30 | Po | 9.44 ± 1.38 | 8.93 | 9.96 | Po | Pnon | 0.47 |
| Co | 0.00001 | |||||||
| Cnon | 0.00001 | |||||||
| 30 | Pnon | 9.23 ± 1.16 | 8.79 | 9.66 | Pnon | Co | 0.00001 | |
| Cnon | 0.00001 | |||||||
| 15 | Co | 4.93 ± 0.75 | 4.51 | 5.35 | Co | Cnon | 0.253 | |
| 15 | Cnon | 5.41 ± 0.78 | 4.97 | 5.84 | ||||
| Note: PO: Patient Obese; Pnon: Patient non obese; Co: Control obese; Cnon: Control non obese, The mean difference is significant at P ≤ 0.05 | ||||||||
Table 2: Fasting serum glucose, HbA1C and BMI in the study subjects.
Determination of monocyte chemoattractant protein 1 (pg/mL)
Table 3 shows that compared to both obese and non-obese individuals, MCP-1 levels are lower in obese patients. However, this difference is not statistically significant. When comparing the levels of Monocyte Chemoattractant Protein 1 (MCP-1) between groups, those with obesity had higher levels than those without obesity (p=0.0001). Likewise, the obese patients contrasted favorably to the non-obese controls (p0.001). Although MCP-1 levels were much higher in the Pnon than in the no obese controls, the difference was not statistically significant (p=0.253). In addition to Pnon vs. Co (p=0.846).
| Parameter | N | Groups | Mean ± SD | CI 95% | Compared groups | P value | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| MCP-1 pg\ml. | 30 | Po | 329.01 ± 117.52 | 285.13 | 372.9 | Po | Pnon | 0.00001 |
| Co | 0.00001 | |||||||
| Cnon | 0.00001 | |||||||
| 30 | Pnon | 100.19 ± 8.97 | 96.84 | 103.54 | Pnon | Co | 0.846 | |
| Cnon | 0.253 | |||||||
| 15 | Co | 95.97 ± 12.57 | 89.01 | 102.93 | Co | Cnon | 0.41 | |
| 15 | Cnon | 75.20 ± 9.12 | 70.15 | 80.25 | ||||
| Note: Po: Patients obese; pnoun: Patient non-obese; Co: Control obess; Cnoun: Control non obese | ||||||||
Table 3: The difference between the ADIPOR1 patient groups and the control groups (ng/mL).
MCP-1 levels have also been linked to atherosclerosis-related problems such as ischemic stroke, myocardial infarction and cardiovascular disease mortality. Obese people appear to have stronger correlation than people of normal weight. High plasma MCP-1 levels have been related with a poor prognosis in progressive heart failure and death after acute coronary syndromes. According to research by Piemonti L, et al., the vast majority of people who develop diabetes have type 2, which is related with excess body fat and IR. Research has shown that those with type 2 diabetes had significantly greater MCP-1 blood levels. Comorbidities associated with obesity are in part caused by blood monocytes participation in cytokine generation, migration into inflammatory adipose tissue, and vascular adhesion. Monocyte Chemoattractant Protein-1 (MCP-1) and other cytokines and chemokines. While there is growing evidence that adiponectin has anti-inflammatory effects, a few in vitro studies have shown otherwise. These studies were conducted in endothelial and monocytic cell lines, and while the precise kind of adiponectin employed and the stimulation settings may make a difference, the findings revealed that adiponectin truly had pro-inflammatory activities. Studies have indicated that adiponectin acts as a local inflammatory regulator in adipose tissue. According to research individuals gain weight, monocyte numbers and activation levels rise, a condition known as diabetes. MCP-1 also has a role in the production of thrombin, the development of clots, the control of tissue factors, and the activity of (PAI-1) [8-14].
Determination of Plasminogen activation inhibitor-1 (ng/ml)
In Table 4 PAI-1 is no significantly reduced in patients obese associated to obese healthy, (p> 0.05). A non-obese patients (p>0.05) respectively. Plasminogen activation inhibitor-1 (PAI-1) level comparison between different groups. PAI-1 was significantly increased in the patient role obese compared to patients no obese, (p=0.00001). While PAI-1 levels were no significant elevated in the Pnon compared to control obese, (p=0.379).
| Parameter | N | Groups | Mean SD | CI 95% | Compared groups | P value | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| SREPENE-1 (PAI-1) ng\ ml | 30 | Po | 6.25 ± 2.34 | 5.37 | 7.13 | Po | Pnon | 0.00001 |
| Co | 0.00001 | |||||||
| Cnon | 0.00001 | |||||||
| 30 | Pnon | 4.03 ± 2.23 | 3.2 | 4.86 | Pnon | Co | 0. 379 | |
| Cno | 0.00001 | |||||||
| 15 | Co | 3.49 ± 0.94 | 2.97 | 4.01 | Co | Cnon | 0.00001 | |
| 15 | Cnon | 0.72 ± 0.16 | 0.63 | 0.81 | ||||
| Note: Po: Patients obese; pnoun: Patient non-obese; Co: Control obess; Cnoun: Control non obese | ||||||||
Table 4: PAI-1 (ng/mL) comparison between patients and healthy subjects.
Leurs P, et al. found a metabolic syndrome and diabetes, plasminogen activator inhibitor. In individuals with type 2 diabetes, circulating PAI-1 levels are elevated, which contributes significantly to prothrombotic and proatherosclerotic alterations. Furthermore, plasma PAI-1 levels are elevated across the insulin resistance continuum, from the metabolic syndrome to prediabetes (a state of reduced glucose tolerance) to diabetes. Because both obese and non-obese animals had elevated plasma PAI-1 levels in these conditions, obesity appears to play a substantial role. PAI-1 was shown to be linked to CVD and diabetes by Lyon C and Hsueh W, et al. which they say is indicative of the prothrombotic and proinflammatory environment present in IR. Plasma PAI-1 is mostly determined by adipose mass and circulating insulin levels; losing weight and increasing exercise are therefore useful strategies for reducing PAI-1. Along with the current suggestions for antiplatelet medication. Important therapies for reducing prothrombotic and proinflammatory states in diabetes include Peroxisome Proliferator-Activated Receptor (PPAR) agonists/ligands and renin angiotensin system inhibitors, as demonstrated by research by Lyon and Koliaki C the part of these agents in prediabetes or the metabolic syndrome is not yet clear. In humans, the mature adipocyte is a major contributor to the blood circulation of PAI-1. When a person is overweight or insulin resistant, their plasma levels of PAI-1 rise, and these elevated levels are positively connected with characteristics of the MS and are good predictors of future risk for cardiovascular disease and type 2 diabetes. Adiponectin and PAI-1 levels and their connection through different parameters in people with MS suggests that PAI-1 plays a role in the development of insulin resistance and obesity and may be a causative relationship between obesity and CVD (Alessi MC, Juhan-Vague I). Both PAI-1 and MCP-1 are preferentially produced from visceral adipose tissue in humans, and visceral adipose mass is a primary determinant of PAI-1 levels. Therefore, the increased PAI-1 and MCP-1 levels reported in fructose consumers may be explained by their increased visceral adipose tissue deposition [15-18].
Measurement of serum lipid profile
Table 5 shows that the levels of TC, TG, and LDL-C were substantially (P0.05) higher in the obese diabetes patients group compared to the no obese diabetic patients group. HDL levels did not differ significantly (P>0.05) between the obese and non-obese diabetes patient groups. As can be shown in Table 5, the mean serum HDL levels of the obese diabetes patients group were lower than the mean levels of the no obese diabetic patients group.
| Parameter | N | Groups | Mean ± SD | CI 95% | Compared groups | P value | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| T.G (mg/dL) | 30 | Po | 292.30 ± 123.88 | 246.04 | 338.56 | Po | Pnon | 0.00001 |
| Co | 0.382 | |||||||
| Cnon | 0.004 | |||||||
| 30 | Pnon | 214.75 ± 46.37 | 197.43 | 232.07 | Pnon | Co | 0.028 | |
| Cnon | 0.0.001 | |||||||
| 15 | Co | 129.81 ± 15.20 | 121.39 | 138.23 | Co | Cnon | 0.422 | |
| 15 | Cnon | 106.90 ± 25.44 | 92.81 | 120.99 | ||||
| T.C (mg/dL) | 30 | Po | 301.32 ± 68.04 | 275.91 | 336.72 | Po | Pnon | 0.00001 |
| Co | 0.00001 | |||||||
| Cnon | 0.00001 | |||||||
| 30 | Pnon | 232.11 ± 27.33 | 221.91 | 242.32 | Pnon | Co | 0.00001 | |
| Cnon | 0.00001 | |||||||
| 15 | Co | 135.00 ± 26.97 | 120.06 | 149.93 | Co | Cnon | 0.558 | |
| 15 | Cnon | 125.14 ± 33.09 | 106.82 | 143.47 | ||||
| HDL (mg/dL) | 30 | Po | 41.58 ± 6.91 | 39 | 44.16 | P | Pnon | 0.006 |
| Co | 0.014 | |||||||
| Cnon | 0.00001 | |||||||
| 30 | Pnon | 46.88 ± 6.42 | 44.48 | 49.28 | Pnon | Co | 0.858 | |
| Cnon | 0.001 | |||||||
| 15 | Co- | 47.39 ± 6.41 | 43.84 | 50.94 | Co | Cnon | 0.009 | |
| 15 | Cnon | 54.57 ± 10.25 | 48.89 | 60.25 | ||||
| LDL (mg/dL) | 30 | Po | 201.27 ± 62.62 | 177.89 | 224.65 | Po | Pnon | 0.00001 |
| Co | 0.00001 | |||||||
| Cnon | 0.00001 | |||||||
| 30 | Pnon | 142.28 ± 25.43 | 132.78 | 151.77 | Pnon | Co | 0.00001 | |
| Cnon | ||||||||
| 15 | Co | 61.64 ± 30.46 | 44.77 | 78.51 | Co | Cnon | 0.435 | |
| 15 | Cnon | 49.19 ± 34.92 | 29.85 | 68.53 | ||||
| vLDL (mg/dL) | 30 | Po | 58.46 ± 24.77 | 49.2 | 67.71 | Po | Pnon | 0.001 |
| Co | 0.00001 | |||||||
| Cnon | 0.00001 | |||||||
| 30 | Pnon | 42.95 ± 9.27 | 39.48 | 46.41 | Pnon | Co | 0.001 | |
| Cnon | 0.00001 | |||||||
| 15 | Co | 25.96 0 ± 3.04 | 24.27 | 27.64 | Co | Cnon | 0.422 | |
| 15 | Cnon | 21.38 0 ± 5.08 | 18.56 | 24.19 | ||||
| Note: Po: Patients obese; pnoun: Patient non-obese; Co: Control obess; Cnoun: Control non obese | ||||||||
Table 5: Lipid profile parameters in patients and control groups.
When compared to the healthy obese group and the healthy no obese group, diabetic obesity persons had significantly higher T.C, LDL, VLDL and T.Gs levels. When compared to the healthy obese and healthy no obese groups, substantial increases in T.C, LDL-C, VLDL-C, and T.Gs levels were recognized in the diabetic no obese patients group. While HDL-C levels show a nonsignificant variation in these four groups. BMI, waist-hip ratio, body fat mass, body fat percentage, and all of the MS parameters were favorably correlated with plasminogen activation inhibitor-1 (PAI-1), whereas Adiponectin was negatively correlated with all of these variables except for HDL, where the trend was inverted. Multiple regression analysis showed that waist hip height ratio and triglyceride levels were independent predictors of adipocytes, and that subjects with MS had lower adiponectin and greater PAI-1 levels than healthy controls. Public health efforts to stop the current epidemic of diabetes and CVD are urgently needed because of the evidence linking changes in lifestyle to improvements in several aspects of MS. Garg, et al.; Ivanova, et al. provide two examples. LDL (-) in type 2 diabetics' plasma was greater than in controls, even after glycemic correction. LDL (-) demonstrated a lower binding affinity to LDLr in cultured fibroblasts than LDL (+), therefore endothelial cells released more MCP-1 and IL-8. Diabetes atherosclerosis is caused by interactions between LDLs and arterial wall cells. LDL and IDL from type 2 diabetics encouraged human endothelial cells to generate MCP-1 mRNA [19-23].
Correlation analysis
The relevance of adiponectin with the biochemical parameters in the diabetic obese patients: Table 6 shows non-significant positive correlations of diabetic obese patients obtained from LH, HDL, FSH, TAC, and ADIPOR1. Non-significant negative correlations were obtained from Age, FBS, BMI, T.G, ADIPO, MDA, LDL, MCP-1, PAI-1, TOS and vLDL.
| Parameters | ADIPO(ng/mL) | |
|---|---|---|
| r | P value | |
| Age (years) | -0.122 | 0.519 |
| BMI (kg/m2) | -0.441 | 0.015 |
| FBG (mg/dl) | -0.898 | 0.00001 |
| T.C (mg/dl) | -0.686 | 0.00001 |
| T.G (mg/dl) | -0.7 | 0.00001 |
| HDL-C (mg/dl) | 0.473 | 0.00001 |
| LDL (mg/dl) | -0.754 | 0.00001 |
| vLDL (mg/dl) | -0.655 | 0.00001 |
| MDA (µmol/L) | -0.509 | 0.004 |
| TAC (µmol/L) | 0.817 | 0.00001 |
| TOS (µmol/L) | -0.179 | 0.341 |
| ADIPOR1 | 0.811 | 0.00001 |
| HbA1C | -0.394 | 0.031 |
| PAI-1 | -0.83 | 0.0001 |
| MCP-1 | -0.641 | 0.0001 |
| FSH | 0.471 | 0.009 |
| LH | 0.24 | 0.201 |
Table 6: The correlation of adiponectin with concentrations of biochemical parameters in the diabetic obese patients.
Fasting blood glucose, adiponectin, and resisting all have a strong inverse correlation with serum adiponectin levels. (Chisquare=0.013; r=-7.9) P=0.019, r =-6.6, significance level d. On the other hand, a significant positive correlation was found between serum resisting and fasting blood glucose. (Chi square=0.015; r=6.0).
The relevance of mcp-1 with the biochemical parameters in the diabetic obese patients: Table 7 shows non-significant negative correlations of diabetic obese patients obtained from HDL, LH, FSH, TAC, and ADIPOR1. Non-significant positive correlations were obtained from Age, FBS, BMI, T.G, ADIPO, MDA, LDL, PAI-1, TOS and vLDL.
| Parameters | MCP-1 (ng/mL) | |
|---|---|---|
| r | P value | |
| Age (years) | 0.251 | 0.181 |
| BMI (kg/m2) | 0.428 | 0.018 |
| FBG (mg/dl) | 0.715 | 0.00001 |
| T.C (mg/dl) | 0.336 | 0.069 |
| T.G (mg/dl) | 0.333 | 0.006 |
| HDL-C (mg/dl) | -0.333 | 0.072 |
| LDL (mg/dl) | 0.703 | 0.00001 |
| vLDL (mg/dl) | 0.436 | 0.016 |
| HbA1C | 0.308 | 0.09 |
| MDA (µmol/L) | 0.398 | 0.029 |
| TAC (µmol/L) | -0.619 | 0.00001 |
| TOS (µmol/L) | 0.199 | 0.291 |
| ADIPOR1 | -0.676 | 0.00001 |
| FSH | -0.364 | 0.048 |
| LH | -0.263 | 0.159 |
| PAI-1 | 0.00001 | |
Table 7: The correlation of MCP-1with concentrations of biochemical parameters in the diabetic obese patients.
The relevance of pai-1 with the biochemical parameters in the diabetic obese patients: Table 8 shows non-significant negative correlations of diabetic obese patients obtained from HDL, LH, FSH, TAC, and ADIPOR1. Non-significant positive correlations were obtained from Age, FBS, BMI, T.G, MDA, LDL, TOS and vLDL.
| Parameters | PAI1-1 (ng/mL) | |
|---|---|---|
| r | P value | |
| Age (years) | 0.304 | 0.102 |
| BMI (kg/m2) | 0.286 | 0.141 |
| FBG (mg/dl) | 0.755 | 0.00001 |
| T.C (mg/dl) | 0.593 | 0.001 |
| T.G (mg/dl) | 0.611 | 0.00001 |
| HDL-C (mg/dl) | -0.342 | 0.064 |
| LDL (mg/dl) | 0.697 | 0.00001 |
| vLDL (mg/dl) | 0.577 | 0.001 |
| HbA1C | 0.534 | 0.002 |
| MDA (µmol/L) | 0.195 | 0.3 |
| TAC (µmol/L) | -0.563 | 0.001 |
| TOS (µmol/L) | 0.238 | 0.204 |
| ADIPOR1 | -0.735 | 0.00001 |
| FSH | -0.452 | 0.012 |
| LH | -0.084 | 0.658 |
Table 8: The correlation of PAI-1with concentrations of biochemical parameters in the diabetic obese patients.
The relevance of ADIPOR1 with concentrations of biochemical parameters in the diabetic obese patients: Table 9 shows non-significant positive correlations of diabetic obese patients obtained from LH, FSH and TAC. Non-significant negative correlations were obtained from Age, FBS, BMI, T.G, ADIPO, HDL, MDA, LDL, MCP-1, PAI-1, TOS and vLDL.
| Parameters | ADIPOR1 (ng/mL) | |
|---|---|---|
| r | P value | |
| Age (years) | -0.065 | 0.73 |
| BMI(kg/m2) | -0.608 | 0.00001 |
| FBG (mg/dl) | -0.831 | 0.00001 |
| T.C (mg/dl) | -0.54 | 0.004 |
| T.G (mg/dl) | -0.637 | 0.00001 |
| HDL-C (mg/dl) | 0.615 | 0.00001 |
| LDL (mg/dl) | -0.792 | 0.00001 |
| vLDL (mg/dl) | -0.554 | 0.001 |
| HbA1C | -0.425 | 0. 019 |
| MDA (µmol/L) | -0.636 | 0.00001 |
| TAC (µmol/L) | 0.66 | 0.00001 |
| TOS (µmol/L) | -0.091 | 0.63 |
| FSH | 0.451 | 0.012 |
| LH | 0.322 | 0.082 |
Table 9: The correlation of ADIPOR1 with concentrations of biochemical parameters in the diabetic obese patients.
Conclusion
Excessive adiposity is the direct cause of type 2 diabetes, and the chance of developing the disease increases in a linear fashion along with body mass index. The increase in the prevalence of type 2 diabetes has so paralleled the worldwide trend toward increasing rates of obesity.
References
- Panee J. Monocyte Chemoattractant Protein 1 (MCP-1) in obesity and diabetes. Cytokine. 2012;60:1-12. [Crossref] [Google Scholar]
- Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med 2007; 13:332–339. [Crossref] [Google Scholar] [PubMed]
- Jung UJ, Choi MS. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15:6184-6223. [Crossref] [Google Scholar] [PubMed]
- van Greevenbroek MM, Schalkwijk CG, Stehouwer CD. Obesity-associated low-grade inflammation in type 2 diabetes mellitus: causes and consequences. Neth J Med. 2013;71:174-87. [Google Scholar] [PubMed]
- Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: Depot difference and regulation by glucocorticoid. J Clin Endocr. 1998;83:847-850. [Crossref] [Google Scholar] [PubMed]
- Speliotes EK, Massaro JM, Hoffmann U, Vasan RS, Meigs JB, Sahani DV, et al. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: The Framingham Heart Study. Hepatology. 2010;51:1979-1987. [Crossref] [Google Scholar] [PubMed]
- Nathan DM, Turgeon H, Regan S. Relationship between glycated haemoglobin levels and mean glucose levels over time. Diabetologia. 2007;50:2239-2244. [Crossref] [Google Scholar] [PubMed]
- Elena P, Paivi H, Heini H, Saara M. Glycaemic control in insulin deficient patients using different insulin delivery and glucose sensoring devices: cross-sectional real-life study. Diabet Epid Manage. 2022; 100072.
- International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009, 32:1327-1334. [Crossref] [Google Scholar] [PubMed]
- Bilgili S, Celebiler AC, Dogan A, Karaca B. Inverse relationship between adiponectin and plasminogen activator inhibitor-1 in metabolic syndrome patients. Endocr Regul. 2008;42:63-68. [Google Scholar]
- Cho NH, Ku EJ, Jung KY, Oh TJ, Kwak SH, Moon JH, et al. Estimated association between cytokines and the progression to diabetes: 10-year follow-up from a community-based cohort. J Clin Endocrinol Metab. 105:381-389. [Crossref] [Google Scholar] [PubMed]
- Nawaz SS, Siddiqui K. Plasminogen activator inhibitor-1 mediate downregulation of adiponectin in type 2 diabetes patients with metabolic syndrome. Cytokine X. 2022;4:100064. [Crossref] [Google Scholar] [PubMed]
- Grant PJ. Diabetes mellitus as a prothrombotic condition. J Intern Med. 2017;262:157-172. [Crossref] [Google Scholar] [PubMed]
- Banerjee A, Pathak S, Duttaroy AK. Dietary Fats and the Gut Microbiota: Their impacts on lipid-induced metabolic syndrome. J Funct Foods. 91:105026-10502. [Crossref] [Google Scholar]
- Leurs PB, Stolk RP, Hamulyak K, Oerle RV, Grobbee DE, Wolffenbuttel BH. Tissue factor pathway inhibitor and other endothelium-dependent hemostatic factors in elderly individuals with normal or impaired glucose tolerance and type 2 diabetes. Diabetes Care, 25:1340-1345. [Crossref] [Google Scholar] [PubMed]
- Lyon CJ, Hsueh WA. Effect of plasminogen activator inhibitor–1 in diabetes mellitus and cardiovascular disease. Am J Med. 2003;115:62-68. [Crossref] [Google Scholar] [PubMed]
- Koliaki C, Liatis S, Kokkinos A. Obesity and cardiovascular disease: revisiting an old relationship. Metabolism. 2019;92:98-107. [Crossref] [Google Scholar] [PubMed]
- Piemonti L, Calori G, Lattuada G, Mercalli A, Ragogna F, Garancini MP, et al. Association between plasma monocyte chemoattractant protein-1 concentration and cardiovascular disease mortality in middle-aged diabetic and nondiabetic individuals. Diabetes care. 2020;32:2105-2110. [Crossref] [Google Scholar] [PubMed]
- Sharma D, Arora S, Banerjee A, Singh J. Improved insulin sensitivity in obese-diabetic mice via chitosan Nanomicelles mediated silencing of pro-inflammatory Adipocytokines. Nanomedicine. 2021;33:102357. [Crossref] [Google Scholar] [PubMed]
- Panee J. Monocyte Chemoattractant Protein 1 (MCP-1) in obesity and diabetes. Cytokine. 2012;60:1-12. [Crossref] [Google Scholar] [PubMed]
- Arakelyan A, Petrkova J, Hermanova Z, Boyajyan A, Lukl J, Petrek M. Serum levels of the MCP-1 chemokine in patients with ischemic stroke and myocardial infarction. Mediators of inflammation, 2005;3:175-179. [Crossref] [Google Scholar] [PubMed]
- Piemonti L, Calori G, Lattuada G, Mercalli A, Ragogna F, Garancini MP, et al. Association between plasma monocyte chemoattractant protein-1 concentration and cardiovascular disease mortality in middle-aged diabetic and nondiabetic individuals. Diabetes Care. 2009;32:2105-2110. [Crossref] [Google Scholar] [PubMed]
- Gupta P, Gupta S. Evaluation of Lipid Profile in Type-II Diabetes Mellitus with Obesity. J Med Sci Clin Res. 2016;88:2455-0450. [Crossref] [Google Scholar]




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.