Lipid and Some Other Cardiovascular Risk Factors Assessment in a Rural Community in Eastern Nigeria
- *Corresponding Author:
- Dr. Gladys Ifesinachi Ahaneku
Department of Medicine, College of Health Sciences, Nnamdi Azikiwe University, P.M.B. 5001, Nnewi, Anambra State, Nigeria.
E-mail: gladysahaneku@yahoo.co.uk
Citation: Ahaneku GI, Ahaneku JE, Osuji CU, Oguejiofor CO, Anisiuba BC, Opara PC. Lipid and some other cardiovascular risk factors assessment in a rural community in Eastern Nigeria. Ann Med Health Sci Res 2015;5:284-91.
Abstract
Background: Continuous re‑evaluation of modifiable cardiovascular risk factors (cardiovascular diseases [CVDs]) in developing nations is imperative as it lays foundation for early preventive/intervention measures at grass root level to improve/prevent CVD morbidity and mortality in those nations where health indices still score below the standard. Aim: The aim was to assess CVD risk factors as a continuous re‑evaluation of these may underscore the need for early intervention measures at grass root level. Subjects and Methods: A total of 257 apparently healthy inhabitants aged 18–85 years were recruited in a rural community in South Eastern Nigeria by convenient sampling. Blood pressure, waist circumference and blood lipid analysis were done procedurally and data analyzed using SPSS 16.0 statistical software. Results: The males were older (59.41 [5.22]) than the females (53.31 [16.90]). 69.2% (133/192) were low level farmers, retirees and dependents. Total cholesterol (TC), low density lipoprotein (LDL), and risk predictive index were higher in females while triglyceride (TG), high density lipoprotein and very LDL (VLDL) were higher in males. The middle aged and elderly respectively had higher TG and VLDL compared to the young. Aside hypertriglyceridemia, all lipid abnormalities were higher in females than males both singly (high TC: 28.9% [35/121] vs. 16.9% [12/71]; high LDL cholesterol: 52.0% [63/121] vs. 31.0% [22/71]) and in combination hypercholesterolemia with hypertriglyceridemia (42.9% [52/121] vs. 36.6% [26/71]). “Multiple risk factors” also occurred more in females with seeming further increase in older age. Conclusion: The chances of a female having CVD after menopause seemed to outweigh that of the male. CVD preventive measures should be focused at the primary/community level as a means to curtailing the increasing morbidity and eventual mortality from CVDs.
Keywords
Blood pressure, Homogenous community, Lipids, Waist circumference
Introduction
The concept of predicting future morbidity and mortality from cardiovascular diseases (CVDs) by measuring such factors as blood pressure (BP), body weight, or index of obesity and blood lipid originated in the life insurance industry during the 1940’s and 1950’s[1] for individuals who sought and obtained life insurance. Hyperlipidemia, hypertension, and possibly obesity (all of which have long time recognized association with one another) appear to be the most important treatable factors that predispose patients to coronary heart disease. Coexistence of these factors is known to have multiplier effect with other CVD risk factors and has continued to translate to increasing CVD morbidity and mortality.
These noncommunicable diseases (NCDs) were some decades ago described as rare or low in blacks but more recent researches in Nigeria and other countries[2‑17] indicate that their incidence in developing countries is gradually taking a prime position.
Bearing this changing trend in mind, continuous re‑evaluation of these CVD risk factors cannot be over emphasized; more so, in different communities in developing nations where health indices still score below the standard. This regular assessment will re‑evaluate data for these variables and emphasize the need for better education and provision of other appropriate early intervention measures at the grass root level. The positive impact of this may be quite enormous especially in a country like Nigeria where quacks and alternative health practitioners are readily available and render their services more or less unchecked. In this study, therefore, lipids and various CVD risk factors were assessed in a rural community in Southern Nigeria whose inhabitants are mainly of low socioeconomic class.
Subjects and Methods
The study was a cross‑sectional community‑based prevalence study carried out in August, 2011 in a homogenous (rural community) in Udi Local Government Area of Enugu State, Southeast Nigeria with a population of about 12,990 (projected at 15% increase every 5 years from 1991 census).
Before commencing the study, approval was obtained from the research and Ethics Committee of the Nnamdi Azikiwe University Teaching Hospital (NAUTH), Nnewi. Letters were written respectively to the traditional ruler and the town union of the community as well as to the local government authority and their written approval obtained. Informed consent was also obtained from each participant before being included in the study.
The sample size was calculated to be approximately 246 using the prevalence of multiple risk factors for coronary vascular disease as found in Ibadan, Nigeria which was 20%[18] using the standard formula. However, a total of 257 inhabitants of the community that participated in this study had their data analyzed.
All consenting apparently healthy subjects 18 years and above residing in the community were recruited into the study. All those with a history of current use of steroids, clinical evidence of fluid retention and all pregnant females were excluded from the study. Six medical officers were trained to help in this study along with two laboratory scientists. General physical examination was carried out on each participant who then had his/her waist circumference (WC) measured with a nonstretchable tape. The umbilicus was the landmark and where the abdomen was pendulous, the point in the abdomen with highest circumference was taken. WC ≥ 102 cm for males and ≥ 88 cm for females was regarded as abdominal obesity. Afterward, the participant got seated and a questionnaire detailing the individuals’ demographic and relevant family and social history was administered to each participant by a trained interviewer. This also afforded him/her opportunity to relax before the BP was checked.
Each participant’s BP was measured using the standard procedure. Three readings were taken at about 5–10 min interval and the mean of the last two was regarded as the subject’s BP. Hypertension was defined as BP ≥ 140/90 mmHg. Ten milliliters of venous blood was withdrawn from willing participants who had not eaten into a container containing dry sodium ethylenediaminetetraacetic acid (1 mg/ml) mixed gently and separated and stored at − 20°C until analysis. The fasting period before sample collection was a minimum of 10 h since their last meal was at night and blood samples were collected before breakfast. Those who came after they had breakfast were asked to come the next day for collection of their fasting blood sample. Plasma total cholesterol (TC), high‑density lipoprotein cholesterol (HDLC) and triglycerides (TGs) were done by colorimeter (enzymatic methods) in the chemical pathology laboratory of NAUTH, Nnewi using diagnostic sera kits by RANDOX Laboratories UK while low‑density lipoprotein cholesterol (LDLC), and very LDLC (VLDLC) were calculated using the Friedewald Formula;[19] thus:
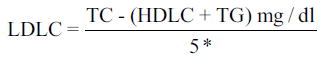
*2.2 if units were expressed in mmol/L

or
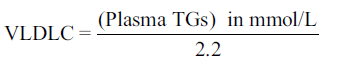
The coronary heart disease risk predictive index was also calculated for each participant as LDLC/HDLC with value < 2.1 as desirable. For each batch of the assay, a commercial control serum of known value was always included and all the parameters were assayed within the same period in order to minimize inter and intra batch errors.
The NAUTH reference ranges were used in interpreting the lipid parameters. Hyperlipidemia was defined as raised plasma TC and/or raised plasma TG that is, TC > 5.17 mmol/L and or TG > 1.71 mmol/L (NAUTH reference ranges). Combined dyslipidemia was defined as TC > 5.17 mmol/L and or TG > 1.71 mmol/L plus low HDL. The lipid values for samples that were not properly labeled were all not included in the analysis. Lipid profile results were later sent to all participants who desired to have their cholesterol results sent to them.
Data analysis
The Microsoft Excel 2003 worksheet and SPSS (16.0) statistical software (manufacturer: SPSS Inc., 233 South Wacker Drive, 11th Floor, Chicago, IL 60606‑6412. Patent No. 7,023,453) were used for data entry, validation, and analysis. Frequency distribution tables were formed from which percentages, mean values and standard deviations of the parameters studied were determined appropriately. Analysis of variance and Student’s t‑test were used to look for gross differences in the parameters among groups of the subjects. P < 0.05 was taken as significant.
Results
The occupations of the participants were farming (56.0% [144/257]), retirees/dependents (13.2% [34/257]), petty trading (7.8% [20/257]), Teaching/other paid jobs (9.3% [24/257]), artisans (7.4% [19/257]), and students (6.2% [16/257]). 27.6% (71/257) of the participants (27.5% [50/181]) of females and (14.5% [11/76]) of males ; P = 0.046 were overweight whi le 12.8% (33/257) (16.0% [29/181] of females and 0.9% [7/76] of males; P = 0.26) were globally obese (body mass index ≥30 kg/m2).
Table 1 shows that the males were significantly older than the females (P < 0.01). The TC and LDL as well as the risk predictive index (RPI) were significantly higher in the females than in the males (P < 0.01, P = 0.02 and P = 0.03, respectively). TG, HDL, and VLDL were higher in the males than the females though not significantly (P = 0.54, 0.26 and 0.92, respectively).
| Parameters | Male(n=76) | Female(n=181) | All subjects(n=257) | P | |
|---|---|---|---|---|---|
| Age (years) | 59.4 (15.22) | 53.31(16.90) | 55.14(16.63) | <0.01* | |
| TG (mmol/L) | 1.22(0.89) | 1.18(0.9) | 1.20(0.89) | 0.54 | |
| TC (mmol/L) | 3.17(1.59) | 3.75(1.45) | 3.58(1.51) | <0.01* | |
| HDL (mmol/L) | 0.39(0.27) | 0.36(0.22) | 0.37(0.23) | 0.26 | |
| VLDL (mmol/L) | 0.56(0.38) | 0.54(0.42) | 0.54(0.41) | 0.92 | |
| LDL (mmol/L) | 2.23(1.52) | 2.85(1.39) | 2.67(1.45) | 0.02* | |
| RPI | 8.00(7.43) | 10.41(7.31) | 9.72(7.41) | 0.03* | |
| BMI (kg/m2) | 24.6 (3.9) | 24.9(5.1) | 24.8 (4.8) | 0.21 | |
| WHR | 0.99(0.07) | 0.95(0.10) | 0.96(0.96) | <0.01 | |
| SBP (mmHg) | 140.29 (31.15) | 137.80 (27.26) | 137.28 (25.96) | 0.84 | |
| DBP (mmHg) | 77.46 | (16.32) | 79.23(14.02) | 78.68(16.62) | 0.64 |
Table 1: Distribution and comparison of age, BP, lipid, and obesity parameters according to gender
As shown in Table 2, apart from HDL which was either the same or slightly lower than the values of the other age groups, all the other lipid parameters and RPI were highest in the middle‑aged subjects whereas HDL was highest in the elderly subjects. Except for TC, LDL, and RPI that were insignificantly higher in the young than in the elderly subjects, all the parameters were lowest in the young subjects. Age differed significantly among the three age groups and within pairs of the different age groups (P < 0.001). Between the young and the elderly, TG, VLDL, waist‑to‑hip ratio, and systolic BP (SBP) differed significantly (P = 0.02, P = 0.02, P < 0.001 and P < 0.001, respectively). Between the young and the middle‑aged, TG as well as SBP and VLDL differed significantly (P = 0.03, P < 0.001, and P = 0.02, respectively). However, between the middle‑aged and the elderly, LDL and RPI were significantly different (respectively P = 0.05 and 0.04).
| Parameters | Age groups (years) | ANOVA (P) | Student’s t‑test (P) | ||||
|---|---|---|---|---|---|---|---|
| <45 (young)(n=64) | 45-64 (middle‑aged)(n=108) | 65+ (elderly)(n=85) | All age | <45 versus65+ | <45 versus45-64 | 45-64versus 65+ | |
| groups | |||||||
| Age (years) | 30.25 (8.71) | 54.4(5.72) | 72.28 (5.85) | <0.001** | <0.001** | <0.001** | <0.001** |
| TG (mmol/L) | 0.93(0.82) | 1.26(0.84) | 1.20(0.89) | 0.04 | 0.02* | 0.03* | 0.58 |
| TC (mmol/L) | 3.41(1.50) | 3.83(1.56) | 3.37(1.41) | 0.17 | 0.12 | 0.83 | 0.08 |
| HDL (mmol/L) | 0.35(0.23) | 0.36(0.25) | 0.38(0.22) | 0.75 | 0.45 | 0.52 | 0.95 |
| VLDL (mmol/L) | 0.42(0.37) | 0.57(0.38) | 0.54(0.45) | 0.04 | 0.02* | 0.03 | 0.59 |
| LDL (mmol/L) | 2.64 1.47) | 2.89(1.52) | 2.40(1.32) | 0.13 | 0.07 | 0.66 | 0.05* |
| RPI | 9.86(7.08) | 10.80 (8.29) | 8.19(6.08) | 0.06 | 0.03* | 0.75 | 0.04* |
| SBP (mmHg) | 126.22 (20.99) | 139.81 (29.08) | 145.21 (27.21) | 0.001* | <0,001* | 0.01* | 0.22 |
| DBP (mmHg) | 91.33(18.23) | 81.46(15.67) | 78.99(14.03) | 0.48 | 0.37* | 0.42 | 0.29 |
| WHR | 0.66(0.34) | 0.77(0.31) | 0.79(0.24) | 0.3* | <0.01* | 0.03* | 0.10 |
Table 2: Distribution and comparison of the parameters among the different age groups
As Table 3 shows, the percentage of males who had hypertension (47.4% [36/76]) was higher than that of the females who had hypertension (41.4% [75/181]) though the difference was not statistically significant (P = 0.67 abdominal obesity was significantly higher [P < 0.001]) among the females (38.1% [69/181]) than among the males (13.2% [10/76]). Compared to the males, females had higher prevalence of both hypercholesterolemia (high TC [P = 0.04] and high LDL [P = 0.02] and hypertriglyceridemia [P = 0.41]) as single entities (high TC: 52/181 28.7% vs. 13/7617.1%; P = 0.04 , High LDLC: 51.9% [94/181] vs. 31.6% [24/76]; P = 0.02) as well as in combination (hypercholesterolemia ± hypertriglyceridemia: 43.1% [78/181] vs. 36.8% [28/76]; [P = 0.19]). Combined dyslipidemia (hypercholesterolemia ± hypertriglyceridemia and low HDL) was also more prevalent among the females (42.0% [76/181] vs. 34.2% [26/76]; P = 0.37).
| Risk factors | All subjects (n=257) (%) | Males (n=76) (%) | Females (n=181) (%) | P |
|---|---|---|---|---|
| Mean age (years) | 55.14 (16.63) | 59.49 (15.22) | 53.31 (16.90) | <0.01* |
| HBP | 111 (43.2) | 36 (47.4) | 75 (41.4) | 0.67 |
| Abdominal obesity | 79 (30.7) | 10 (13.2) | 69 (38.1) | <0.001* |
| Hypertriglyceridemia | 63 (24.5) | 20 (26.3) | 43 (23.8) | 0.28 |
| Hypercholesterolemia (high TC) | 65 (25.3) | 13 (17.1) | 52 (28.7) | 0.04* |
| High LDLC | 118 (45.9) | 24 (31.6) | 94 (51.9) | 0.02* |
| Hypercholesterolemia ± hypertriglyceridemia | 106 (41.4) | 28 (36.8) | 78 (43.1) | 0.19 |
| Low HDLC | 248 (96.5) | 71 (93.4) | 177 (97.8) | 0.27 |
| Combined dyslipidemia (hypercholesterolemia ± | 102 (39.7) | 26 (34.2) | 76 (42.0) | 0.37 |
| hypertriglyceridemia and low HDL) | ||||
| CVD RPI | 234 (91.1) | 62 (81.6) | 172 (95.0) | 0.27 |
Table 3: Prevalence of HBP, abdominal obesity, and lipid abnormalities in relation to gender
Among the subjects that had only one risk factor, there were more males (39.5% [30/76]) than females (34.3% [62/181]). Conversely, for those who had two or more risk factors, there were more females (30.9% [56/181]) than males (21.1% [16/76]).
The differences were, however, not significant (P = 0.20) as shown in Table 4a.
| Number of risk factors | Allsubjects(%) (n=257) | Males(%)(n=76) | Females(%)(n=181) | P |
|---|---|---|---|---|
| Only one risk factor | 92 (35.8) | 30 (39.5) | 62 (34.3) | 0.17 |
| Two or more risk factors | 72 (28.0) | 16 (21.1) | 56 (30.9) | 0.20 |
| Total | 257 (100) | 76 (29.6) | 181 (70.4) |
Table 4a: Number of risk factors according to gender
Those 55 years and above had higher prevalence of “two or more” risk factors (30.7% [42/137]) compared to those < 55 years (25.0% [32/120]; P = 0.37). For those < 55 years, the prevalence of “only one” risk factor was higher in males than the females but “two or more risk factors” was more prevalent in females than the males. However, in those 55 years and above, females had a higher prevalence of both “only one” and “two or more” risk factors [Table 4b].
| Number ofrisk factors | <55 years | >55 years | <55 versus >55 (P) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Males (%)(n=23) | Females (%) (n=97) |
All subjects (%)(n=120) |
Males (%)n=54 |
Females (%) (n=83) |
All subjects (%)(n=137) | ANOVA | Student’s t‑test
|
|||
| <55 | >55 | |||||||||
| All versus all | Male versusfemale | Male versusfemale | ||||||||
| Only onerisk factor | 12 (54.5) | 31 (31.6) | 43 (35.8) | 18 (33.3) | 31 (37.3) | 49 (35.8) | 0.19 | 0.03* | 0.41 | |
| Two or morerisk factors | 3 (13.6) | 27 (27.6) | 30 (25.0) | 13 (24.1) | 29 (34.9) | 43 (30.7) | 0.37 | 43 | 0.79 | |
Table 4b: Number of risk factors in those below and above 55 years in relation to gender
Table 5 shows the number of risk factors in the young, middle aged and elderly subjects. Among the three age groups, the elderly subject had highest prevalence of “only one” risk factor followed by the young and then the middle aged subjects. The variations in the prevalence showed no statistical significance (P = 0.53 and 0.53, respectively). However, among the subjects that had “two or more” risk factors, the prevalence was highest in the middle aged subjects compared to the other age groups and this difference was significant between the middle aged and the young subjects (P = 0.05).
| Number of risk factors | Age groups (years) (n=257) | ANOVA (P) | Student’s t‑test (P) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <45 (young) | 45-64 (middle‑aged) | 65+ (elderly) | All age | <45 versus | <45 versus | 45-64 versus | ||||
| (n=56) (%) | (n=115) (%) | (n=86) (%) | groups | 65+ | 45-64 | 65+ | ||||
| Age (years) | 30.25 (8.71) | 54.4 (5.72) | 72.28 (5.85) | <0.001* | <0.001* | <0.001* | <0.001* | |||
| Only one risk factor | 20 (35.5) | 39 (33.9) | 33 (38.4) | 0.53 | 0.26 | 0.17 | 0.83 | |||
| Two or more risk factors | 9 (16.1) | 39 (33.9) | 24 (27.9) | 0.53 | 0.14 | 0.05* | 0.70 | |||
Table 5: Number of risk factors in the young, middle aged and elderly subjects
As Table 6 shows, the hypertensive subjects were significantly older than the nonhypertensive subjects (P < 0.01). All the lipid parameters with the exception of HDL were higher in the hypertensive subjects than in nonhypertensive subjects.
| Parameters | Hypertensive subjects (n=111) | Nonhypertensive subjects (n=146) | Total (n=257) | P |
|---|---|---|---|---|
| Age (years) | 58.69(12.73) | 52.30(18.74) | 55.04(16.70) | ≤0.01* |
| TG (mmol/L) | 1.34 (0.95), n=88 | 1.10 (0.84), n=111 | 1.21 (0.90), n=199 | 0.09 |
| TC (mmol/L) | 3.76(1.63) | 3.43(1.40) | 3.58(1.51) | 0.37 |
| HDL (mmol/L) | 0.36(0.23) | 0.37(0.24) | 0.36(0.23) | 0.18 |
| VLDL (mmol/L) | 0.61(0.43) | 0.50(0.38) | 0.55(0.41) | 0.42 |
| LDL (mmol/L) | 2.80(1.56) | 2.56(1.36) | 2.67(1.45) | 0.43 |
| RPI | 10.56 (8.15) | 9.14(6.84) | 9.77(7.46) | 0.84 |
Table 6: Comparison of the parameters between hypertensive (BP ≥140/90 mmHg) and nonhypertensive subjects
As shown in Table 7, the percentages of those with abdominal obesity who had high BP (HBP) (58.2% [46/79]) differed significantly (P = 0.04) when compared with 36.5% (65/178) who had HBP among those with normal WC. The prevalence of hypercholesterolemia among those with abdominal obesity was significantly higher than among those with normal WC (41.8% [33/79] vs. 18.0% [32/178]; P = 0.03).
| Risk factors | Abdominal obesity (%) (n=79) | Normal WC (%) (n=178) | Total (%) (n=257) | P |
|---|---|---|---|---|
| HBP | 46 (58.2) | 65 (36.5) | 111 (43.2) | 0.04* |
| Hypercholesterolemia | 33 (41.8) | 32 (18.0) | 65 (25.3) | 0.03* |
| HBP (%) (n=111) | Normal BP (%) (n=146) | |||
| Hypercholesterolemia | 31 (27.9) | 34 (23.3) | 65 (25.3) | 0.27 |
| Combined dyslipidemia | 46 (41.4) | 56 (38.4) | 102 (39.7) | 0.14 |
| Dyslipidemia (%) (n=102) | Normolipidemia (%) (n=155) | |||
| HBP | 46 (45.1) | 65 (41.3) | 111 (43.2) | 0.14 |
| Abdominal obesity | 37 (36.3) | 42 (27.1) | 79 (30.7) | 0.73 |
Table 7: Relationship between the various risk factors
Subjects with HBP had higher prevalence of both hypercholesterolemia (27.9% [31/111]) and combined dyslipidemia (41.4% [46/111]) compared to those with normal BP who had high TC (23.3% [34/146]) and combined dyslipidemia (38.4% [56/146]); the difference between these prevalence values was, however, not statistically significant (P = 0.27 and 0.14, respectively). Those with combined dyslipidemia had higher prevalence of hypertension (45.1% [46/102] vs. 41.3% [65/155]) and abdominal obesity (36.3% [37/102] vs. 27.1% [42/15]) compared to those without dyslipidemia.
Discussion
The mean values of the atherogenic lipids were similar to the finding in many other recent studies[9,14‑17] in being lower than the upper limits just as the mean HDL value was lower than the desirable minimum. When compared with those previous local studies, the values obtained in this study were generally much lower. This may be because even when the participants had similar age brackets, most of those previous studies were done in settings and socioeconomic classes that were mixed or different from this one. The participants in this study were mostly low‑level famers, petty trades, and elderly dependents. Thus, as serum lipids are known to be influenced by nutrition, the lower mean lipid values obtained in this study may suggest that the inhabitants of this community had poor/ poorer nutrition
Some previous researchers[8‑10,12,14,15] observed higher values of TG in males than females just as found in this study. However, contrary to some of these studies[10] and in agreement with others,[9,14] the difference in mean TG value in both sexes was not statistically significant. Again, contrary to the finding of a recent study of elderly subjects in south‑east Nigeria,[14] in which males had higher values of all lipids parameters measured, the females in this study had significantly higher TC and LDL values (P < 0.01 and P = 0.02, respectively) than their male counterparts. The observed sex variation in respect of lipid in this and the other study may be accounted for by the differences in the diets of the two different study populations. The findings in this study, however, agrees with the finding of the Nigeria National NCD survey report on cholesterol values in adult males and females.[20]
Whereas older studies observed that serum lipid concentration did not alter with age in underprivileged Africans after adulthood,[21] more recent studies[14] found an age‑related trend in serum lipid concentrations. This study, however, showed no consistent trend. This inconsistent trend supports the finding by Miller[22] in which LDL values showed no consistent trend with age and just like in this study, the 45–64 years age group in their study had the highest values of the parameters measured. The finding of lowest concentration of TC in the elderly (above 65 years) subjects of this study may not necessarily be a contradiction or concordance of earlier studies[23] (in which TC did not necessarily rise with age but TG did) since those earlier studies involved only young and middle age (45–65 years) subjects and thus did not compare the TC values in older age groups as done in this present study. Except for HDL which rose steadily with age, all the other lipid parameters rose from young to middle age and then dropped in the elderly. This observed age‑related variation in lipid level in this study has been demonstrated in different European communities which found that lipid parameters attained peak values at about 50 years after which the values began to fall.[14,24] All the atherogenic risk factors as well as RPI (i.e., LDL/HDL) were more favorable in those who were normotensive and/or “not obese” than in those who had either of these and this is in keeping with other studies [11,25] that reported direct association between lipid levels and LDL/HDL ratio.
The general prevalence of HBP documented in this study was 43.2% (males: 47.4%, females: 41.4%) while abdominal obesity prevalence was 30.7% (males: 13.2%, females: 38.1%). Those with abdominal obesity had significantly higher prevalence of HBP (58.2% vs. 36.5%; P = 0.04) and high TC (41.8% vs. 18.0%; P = 0.03) than those without abdominal obesity; an association that has long been established.[2‑6,26,27] Those with combined dyslipidemia also had a higher prevalence of both HBP (45.1% vs. 41.3%) and abdominal obesity (36.3% vs. 27.1%) than those with normolipidemia, as documented in other studies.[8,9,26,28] In agreement with previous studies which demonstrated high dyslipidemia prevalence in hypertensive patients in Nigeria, mean values of all atherogenic lipids were higher in those found to have hypertension in this study compared to normotensive subjects. Mean HDL value in this study was similar in those with HBP (0.36 [0.23] mmol/L) and in nonhypertensive subjects (0.37 [0.24] mmol/L) thus, collaborating the finding by some researchers that aside HDL every other cardiovascular co‑morbidity was higher in hypertensives.[26]
In this study, 35.8% of the participants (males: 39.5%, females: 34.3%); (P = 0.17) had “only one” of the three cardiovascular risk factors assessed while 28.0% (males: 21.1%, females: 30.9%) had “two or more” risk factors. This finding is higher than the prevalence of multiple risk factors for coronary vascular disease as found over a decade ago in a rural community in Ibadan, Western Nigeria[18] which was 20% and even a more recent study which found prevalence of at least one CVD risk factor to 12.9%. Despite some sociocultural differences between that rural community and this one, our findings suggest that coronary vascular risk factors are not just increasing in Nigerians as single entities but in groups/multiples.
The prevalence of elevated serum TC was 25.3% (males: 17.1%, females: 28.7%), elevated LDLC was 45.9% (males: 31.6%, females: 51.9%) while that of combined hyperlipidemia (high TC and/or high TG level) was 39.7% (males; 34.2%, females; 42.0%). These prevalence values for high TC and high LDL varied with findings by other researchers in Nigeria[8,9,14,16,28] and some other developing nations;[12,13] being lower in some and higher in others. This difference may be accounted for by the fact that those other studies were conducted in settings different from that in which this study was conducted in that those ones were either hospital‑based or involved people of different or mixed socioeconomic class unlike the case in our study.
Low HDL prevalence in this study was as high as 96.5% while combined dyslipidemia was 39.7%; both being higher than previous research findings in Nigerians[8,9,14,15,28] and some Asian countries.[29] On the same note, hypertriglyceridemia prevalence was higher in this study (24.5%) compared to previous research findings in different parts of Nigeria[8,9,15,26,28,30] and South Asia[29] but lower than the finding in one study in Iran.[12] It is, however, similar to the finding in a Lagos; commercial city in Nigeria [8] and in a South African study.[13] Contrary to high LDL which was said to be the most common lipid abnormality followed by LDL, this study found the reverse with low HDL being the most common lipid abnormality before LDL. This opposite finding may be because that was a review study[8] which include studies done in healthy people as well as those done on patients. The finding of low HDL as the most prevalent lipid abnormality has been demonstrated in other community‑based studies.[9,15,28‑30] Hypertriglyceridemia was also the least occurring lipid abnormality as found in some other study, though hospital based.[31] In relation to gender, the females had higher prevalence of high TC (28.7% vs. 17.1%; P < 0.05) and low HDL (97.8% vs. 93.3%) as found in other studies[9,12,15] but contrary to some studies[31] and in agreement with some,[29] the higher low HDL prevalence in females showed no statistical significance compared to the males; P > 0.05. Unlike in one of those studies,[9] this study found that the prevalence of high LDL was still higher in females (51.9% vs. 31.6%; P < 0.05) like in some others.[15]
In the general population, the prevalence of “only one risk factor” in this study was the same both above and below 55 years, whereas “two or more risk factors” was more prevalent above 55 years. Furthermore, the middle aged subjects had highest prevalence of “two or more risk factors” (33.9%) compared to the elderly (27.9%; P = 0.70), and then the young who had the least prevalence (16.1%; P = 0.05). It, therefore, seemed that the prevalence of multiple risk factors increased with age and then began to drop after the middle age in this community; a trend which recent studies have demonstrated for individual CVDs in Nigerians.[2,32]
As can be seen in Table 3, aside hypertriglyceridemia, the females had higher prevalence of all other lipid abnormality compared to the males and as shown in Table 4a and b, they also had higher prevalence of multiple risk factors both in the general population and above and below 55 years age groups. A study conducted about a decade ago in Nigerians[3] indicated that gender was not a modifier of cardiovascular risk in Nigeria. Our data tend to suggest that CVD risk prevalence was higher in females and the degree of risk in them tended to increase further in older age when all the risk factors were considered either singly (“only one risk factor”) or collectively (“two or more risk factors”). These findings suggest that the females in this study (mean age: 53.1 years) seemed to run higher risk of CVD than the males who were significantly older (mean age: 59.4 years; P < 0.01).
Limitations of study
Due to financial constraint and other logistics, contact with each participant was once. Those with grade 1 hypertension should have had follow‑up checks but this first time and only visit was taken as their BP as in other epidemiologic studies.
Conclusion
Cardiovascular disease risk factors are prevalent even in rural communities in Southeast Nigeria and the chances of a female having CVD after menopause seemed to outweigh that of the age‑matched male. There is a need for health education at the primary/community level as a means to curtailing the increasing morbidity and eventual mortality from CVDs.
Recommendation
Periodic screening of individuals at risk by clinicians and other health workers using the parameters studied in this research work is advocated. Education and lifestyle modification are important measures to be deployed by responsible health professionals in addressing the rising trend of CVDs in Nigeria and nations with similar setting.
References
- Society of Actuaries. Build and Blood Pressure Study. Compiled and Publisher by Society of Actuaries, South La salle Street, Chicago 4, Illinois, October, 1959. Printed in USA by Peter F. Mallon Inc. Long Island City, New York. N. Y.
- Ezeoma IT, Abioye‑Kuteyi EA, Oladeji AO. Body build and blood pressure in a rural Nigerian community. Niger Postgrad Med J 2001;8:140‑4.
- Lawoyin TO, Asuzu MC, Kaufman J, Rotimi C, Owoaje E, Johnson L, et al. Prevalence of cardiovascular risk factors in an African, urban inner city community. West Afr J Med 2002;21:208‑11.
- Oghagbon EK, Okesina AB, Biliaminu SA. Prevalence of hypertension and associated variables in paid workers in Ilorin, Nigeria. Niger J Clin Pract 2008;11:342‑6.
- Njelekela MA, Mpembeni R, Muhihi A, Mligiliche NL, Spiegelman D, Hertzmark E, et al. Gender‑related differences in the prevalence of cardiovascular disease risk factors and their correlates in urban Tanzania. BMC Cardiovasc Disord 2009;9:30.
- van der Sande MA, Milligan PJ, Nyan OA, Rowley JT, Banya WA, Ceesay SM, et al. Blood pressure patterns and cardiovascular risk factors in rural and urban gambian communities. J Hum Hypertens 2000;14:489‑96.
- Ahaneku JE, Ndefo JC, Dioka CE. Serum cholesterol level in a typical suburban commercial community in Nigeria. Experientia 1996;52:680‑2.
- Okafor CI. The metabolic syndrome in Africa: Current trends. Indian J Endocrinol Metab 2012;16:56‑66.
- Sani MU, Wahab KW, Yusuf BO, Gbadamosi M, Johnson OV,Gbadamosi A. Modifiable cardiovascular risk factors among apparently healthy adult Nigerian population ‑ A cross sectional study. BMC Res Notes 2010;3:11.
- Adegoke OA, Adedoyin RA, Balogun MO, Adebayo RA, Bisiriyu LA, Salawu AA. Prevalence of metabolic syndrome in a rural community in Nigeria. Metab Syndr Relat Disord 2010;8:59‑62.
- Azinge EC, Sofola OA, Silva BO. Relationship between salt intake, salt‑taste threshold and blood pressure in Nigerians. West Afr J Med 2011;30:373‑6.
- Esteghamati A, Meysamie A, Khalilzadeh O, Rashidi A, Haghazali M, Asgari F, et al. Third national Surveillance of Risk Factors of Non‑Communicable Diseases (SuRFNCD‑2007) in Iran: Methods and results on prevalence of diabetes, hypertension, obesity, central obesity, and dyslipidemia. BMC Public Health 2009;9:167.
- Thorogood M, Connor M, Tollman S, Lewando Hundt G, Fowkes G, Marsh J. A cross‑sectional study of vascular risk factors in a rural South African population: Data from the Southern African Stroke Prevention Initiative (SASPI). BMC Public Health 2007;7:326.
- Odenigbo CU, Odenigbo UM, Oguejiofor OC, Okonkwo UC, Oguanobi NI. Prevalence of dyslipidaemia in elderly subjects in Asaba, South East Nigeria. J Indian Acad Geriatr 2010;6:160‑4.
- Odenigbo CU, Oguejiofor OC, Odenigbo UM, Ibeh CC, Ajaero CN, Odike MA. Prevalence of dyslipidaemia in apparently healthy professionals in Asaba, South East Nigeria. Niger J Clin Pract 2008;11:330‑5.
- Jisieike‑Onuigbo NN, Unuigbe EI, Kalu OA, Oguejiofor CO, Onuigbo PC. Prevalence of dyslipidemia among adult diabetic patients with overt diabetic nephropathy in Anambra state South‑East Nigeria. Niger J Clin Pract 2011;14:171‑5.
- Odenigbo UM, Odenigbo CU, Oguejiofor OC, Oguejiofor CB. Prevalence of metabolic syndrome in healthy professionals in Asaba, South East Nigeria. J Biomed Invest 2009;7:5‑10.
- Ezenwaka CE, Akanji AO, Akanji BO, Unwin NC, Adejuwon CA. The prevalence of insulin resistance and other cardiovascular disease risk factors in healthy elderly southwestern Nigerians. Atherosclerosis 1997;128:201‑11.
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‑density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499‑502.
- Akinkugbe OO. Non‑Communicable Diseases in Nigeria; Final Report of a National Survey. Lagos: Federal Ministry of Health and Social Services; 1997. p. 12‑41.
- Antonis A, Bersohn I. Serum‑triglyceride levels in South African Europeans and Bantu and in ischaemic heart‑disease. Lancet 1960;1:998‑1002.
- Miller GJ, Beckles GL, Alexis SD, Byam NT, Price SG. Serum lipoproteins and susceptibility of men of Indian descent to coronary heart disease. The St James Survey, Trinidad. Lancet 1982;2:200‑3.
- Ononogbu IC. Serum cholesterol levels in a Nigerian population sample. Experientia 1979;35:1428‑9.
- B Lewis, A Chait, M Mancini, LA Carlson, P Oriente, H Micheli, et al. Serum lipoproteins in four European communities: A quantitative comparison. Eur J Clin Invest 1978;8 (3):165‑73.
- Igwe JC, Aloamaka CP, Mgbo N. HDL‑LDL ratio: Asignificant predisposition to the onset of atherosclerosis. Niger J Health Biomed Sci 2003;2:78‑82.
- Ulasi II, Ijoma CK, Onodugo OD. A community‑based study of hypertension and cardio‑metabolic syndrome in semi‑urban and rural communities in Nigeria. BMC Health Serv Res 2010;10:71.
- Ekore RI, Ajayi IO, Arije A. Case finding for hypertension in young adult patients attending a missionary hospital in Nigeria. Afr Health Sci 2009;9:193‑9.
- Akintunde AA, Ayodele EO, Akinwusi OP, Opadijo GO. Dyslipidemia among newly diagnosed hypertensives: Pattern and clinical correlates. J Natl Med Assoc 2010;102:403‑7.
- Flowers E, Molina C, Mathur A, Prasad M, Abrams L,Sathe A, et al. Prevalence of metabolic syndrome in South Asians residing in the United States. Metab Syndr Relat Disord 2010;8:417‑23.
- Oladapo OO, Salako L, Sodiq O, Shoyinka K, Adedapo K, Falase AO. A prevalence of cardiometabolic risk factors among a rural Yoruba south‑western Nigerian population: A population‑based survey. Cardiovasc J Afr 2010;21:26‑31.
- Ogbera AO. Prevalence and gender distribution of the metabolic syndrome. Diabetol Metab Syndr 2010;2:1.
- Isezuo SA, Sabir AA, Ohwovorilole AE, Fasanmade OA. Prevalence, associated factors and relationship between prehypertension and hypertension: A study of two ethnic African populations in Northern Nigeria. J Hum Hypertens 2011;25:224‑30.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.