Lymphatic Drainage of the Deep Inferior Epigastric Artery Perforator Flap after Breast Reconstruction
2 Multi-disciplinary Clinic of Lymphology, Jules Bordet Institute, Brussels, Belgium, Email: pierre525@gmail.com
3 Department of Plastic Surgery, Jules Bordet Institute, Brussels, Belgium, Email: pierre525@gmail.com
4 Service of Nuclear Medicine, Jules Bordet Institute, Brussels, Belgium
Citation: Roman MM, et al. Lymphatic Drainage of the Deep Inferior Epigastric Artery Perforator Flap after Breast Reconstruction. Ann Med Health Sci Res. 2017; 7: 72-75
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: Transplanted tissue in breast reconstruction is routinely performed without any lymphatic anastomosis. Edemas might be observed at the level of these transplanted tissues. New lymphatic routes are supposed to develop but little is known about the development of new lymphatic vessels in the transplanted tissue after free flap transfer. The aim of the study was to evaluate the lymphatic drainage of the skin flap in patients after breast reconstruction. Material and Methods: In a series of 17 breast cancer patients, lymphatic drainage of DIEAP flaps was studied with lymphoscintigraphy. Three groups were differentiated in our series: 6 primary breast reconstructions (in the same operating time as the mastectomy), 9 secondary (in a different operating time as the mastectomy) and 2 for relapsing breast cancer. Results: After injection in the “areola” we noticed ipsilateral axillary and parasternal lymphatic drainage in five patients in the group of 6 while after injection was performed in the median part of the “large” skin flap we noticed ipsilateral axillary and parasternal lymphatic drainage in 7 patients and contralateral axillary and parasternal lymphatic drainage in 5 patients in the group of 9. In the group of 2 patients after injection in the “areola” we noticed only ipsilateral parasternal lymphatic drainage. Conclusions: Our results suggest a decrease in the deep lymphatic drainage of the skin flap (toward the ipsi and contralateral parasternal lymph nodes) in patients after reconstruction with deep flap. These results might have implications in the management of patients especially if edema is observed at the level of the transplanted skin.
Keywords
Lymphatic drainage; Breast cancer; Reconstruction; Deep inferior epigastric artery perforator flap; Lymphoscin
Introduction
In recent times, scientific interest in evaluating the lymphatic drainage of the breast after treatment has increased. [1-3] Even 30 years ago, lymphoscintigraphy of the anterior chest wall was performed to demonstrate changes in lymphatic drainage after extended and/or radical mastectomy, with or without radiotherapy, in breast cancer patients. [4] The problem of the lymphatic drainage is also a matter of concern in women who develop upper limb edema (LE) after mastectomy with axillary node dissection and/or irradiation [5,6] Upper limb lymphedema (LE) represents a common complication after breast cancer treatment. [7] Lymphoscintigraphies have been largely used to study the lymphatic drainages of these oedematous situations. [8-11] However, some patients will be troubled by breast oedema after breast-conserving surgery, followed by radiotherapy. [12] The oedema of the reconstructed breast after DIEP is not clearly illustrated in literature, especially concerning lymphatic drainage. The transfer of vascularized lymph node flaps is a new and encouraging technique for the treatment of postoperative lymphedema of the upper limb. [13] However the lymphatic drainage of the DIEAP itself remains unknown especially, when an oedema is present. The aim of the study was to evaluate the lymphatic drainage of the skin flap in patients after breast reconstruction.
Material and Methods
This retrospective review of data was authorized by our institutional ethical committee (number 2048).
We reviewed the lymphoscintigraphic investigations performed in 17 patients (median age, 50 years; range, 36-62 years) who had undergone, in our institution between August 2006 and November 2011, breast reconstruction with a DIEAP flap (median delay between the breast reconstruction and the scintigraphic exam was 6 months) and who presented objective and/or subjective symptoms of lymphedema at the level of the reconstructed breast and/or at the level of the ipsilateral upper limb [Table 1].The patients were divided into three groups. Patients in the first group (n=6) were received immediate breast reconstruction and patients in the second group (n=9) had delayed breast reconstruction. Two patients were operated on at the time of recurrence of breast cancer, but had the same characteristics as patients in the second group.
| Patients | Side | ALND | RT | Age | Lymphatic drain of the flap | Lymphedema | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 | IA | IP | CP | CA | DF | NMF | ULE | ||||
| 1 | R | Level I-II | No | 44 | Yes | Yes | No | No | No | Yes | No |
| 2 | R | Level I-II | No | 50 | Yes | Yes | No | No | Yes | No | No |
| 3 | L | ND | No | 51 | Yes | Yes | No | No | Yes | No | No |
| 4 | R | SN | No | 52 | No | No | No | No | Yes | Yes | No |
| 5 | L | SN | No | 50 | Yes | yes | No | No | Yes | Yes | No |
| 6 | R | SN | No | 61 | Yes | Yes | No | No | No | No | No |
| Group 2 | |||||||||||
| 1 | R | Level I-II | Yes | 44 | Yes | No | No | No | No | Yes | No |
| 2 | R | Level I-II | Yes | 62 | No | No | No | Yes | Yes | No | No |
| 3 | L | Level I-II | Yes | 55 | Yes | Yes | No | No | No | No | No |
| 4 | L | Level I-II | Yes | 42 | No | No | No | Yes | No | No | Yes |
| 5 | R | Level I-II | Yes | 42 | No | No | Yes | No | No | No | No |
| 6 | R | Level I-II | Yes | 51 | No | Yes | No | Yes | Yes | No | No |
| 7 | L | Level I-II | Yes | 36 | Yes | No | No | Yes | No | No | Yes |
| 8 | L | Level I-II | Yes | 51 | Yes* | No | No | No | No | No | No |
| 9 | R | Level I-II | Yes | 50 | Yes | No | No | No | Yes | Yes | No |
| Group 3 | |||||||||||
| 1 | R | Level I-II | Yes | 48 | No | Yes | No | No | No | Yes | No |
| 2 | R | Level I-II | Yes | 46 | no | Yes | No | No | No | Yes | No |
Abbreviations:R: Right, L: Left, ALND: Axillary Lymph Node Dissection, ND: Not Determined, SN: Sentinel Node, RT: Radiotherapy, IA:Ipsilateral Axillary, IP:Ipsilateral Parasternal, CP: Contralateral Parasternal, CA: Contralateral Axillary, DF:Dieap Flaps, NMF= Native Mastectomy Flaps, ULE: Upper Limb Lymphedema.
*Only lymphatic duct without lymph node.
Table 1: Patients’ data.
All included patients underwent a) a lymphoscintigraphy of the upper limbs (performed according to a standard procedure) [14] followed by b) another investigation of the reconstructed breast, either immediately or later, for which one intradermal injection of 99mTc-labelled human serum albumin (HSA) nanosized colloids (Nanocoll®) (0.4 ml × 0.1 mg × 2 mCi) was given either in the areolar (n=8) or medial (n=9) part of the skin pedicle of the DIEAP flap. Imaging of the lymphatic drainage of the flap was performed after massaging the injected site for a short period of time.
Results
After injecting radioactive tracer in the skin pedicle of the DIEAP flap, we observed lymphatic drainage towards a total of 23 lymphatic basins; ipsilateral axillary drainage in ten cases, ipsilateral parasternal drainage in nine cases, contralateral parasternal drainage in one case, and contralateral axillary drainage in four cases [Table 1].
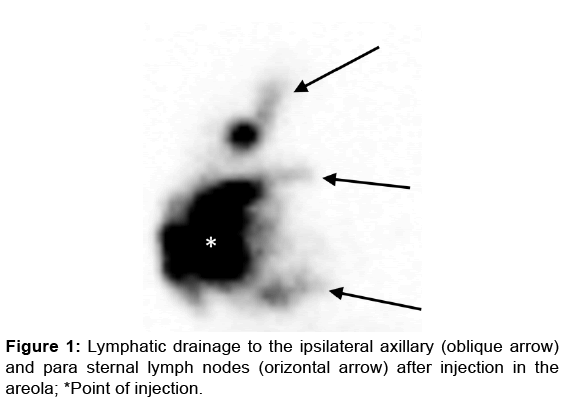
In the group of 6 patients with a primary breast reconstruction in whom the intradermal injection was performed in the areola, no lymphatic drainage was observed in one patient and ipsilateral axillary and parasternal lymphatic drainage was observed in five patients [Figure 1].
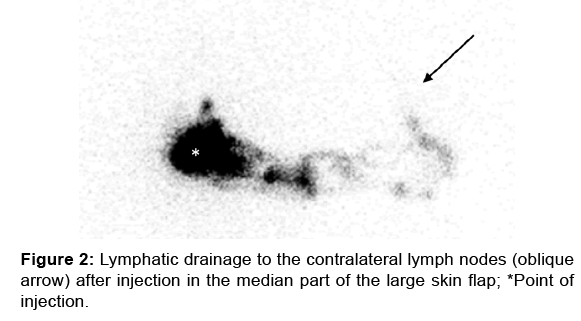
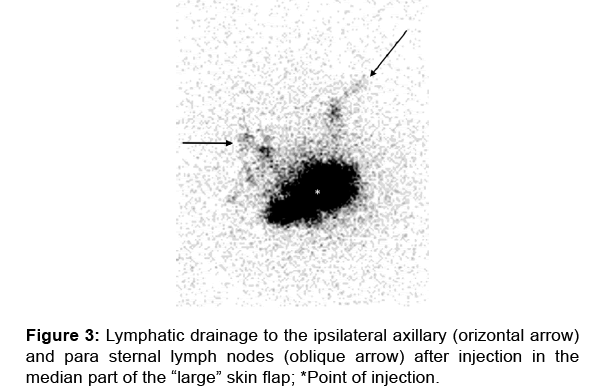
In the group of 9 patients with secondary breast reconstruction in whom the intradermal injection was performed in the median part of the large skin flap, the scintigraphic investigation in 5 patients demonstrated one case with ipsilateral axillary lymphatic drainage with one who also had ipsilateral parasternal drainage [Figure 2]. One patient had only ipsilateral parasternal drainage. Among the 3 patients in whom no ipsilateral lymphatic drainage was observed, two showed drainage toward the contralateral axillary basin [Figure 3] and one toward the contralateral parasternal basin. These three patients with only contralateral drainage had the longest interval between the reconstruction and the scintigraphic exam.
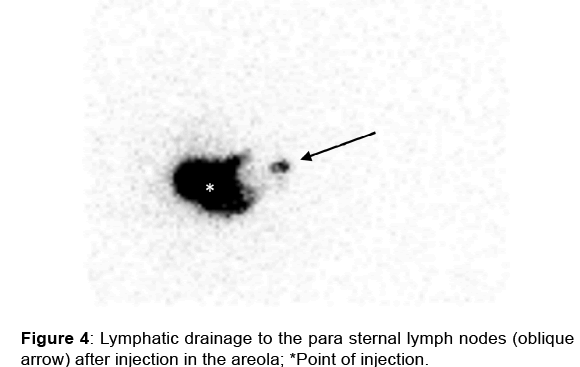
The two patients who had one DIEAP flap at the time of relapse and for whom the intradermal injection was performed in the areola showed only ipsilateral parasternal drainage [Figure 4].
Lymphatic drainage (towards the axillary and/or parasternal ipsilateral basins) was significantly (p < 0.05 using Fisher test) higher in patients who had the injection in the areolar flap (12/16) than in those who had the injection in the median part of the skin flap (7/18).
Discussion
Free perforator flaps like the DIEAP flap are routinely used in breast reconstruction. Arterial and venous micro anastomoses are performed between perforator flap vessels and receptor vessels situated at the thorax, but no lymphatic anastomosis is performed. Oedema, necrosis, seroma formation, and extensive swelling can compromise any free flap. [15] In addition, DIEAP flap are now combined with transfer in the axilla of vascularized lymph node flap to treat lymphedema of the upper limb. [16] However, the mechanism of action of the vascularized lymph node flap, including the drainage of lymph inside the transferred flap, has not yet been firmly established. [13,17] According to Alan et al, [18] tissue transfer can be used to bypass damaged lymphatics and promote rapid lymphatic regeneration. More recently, Tammela et al. demonstrated that lymph node transfer combined with VEGF-C administration after lymphadenectomy in mice can promote reconstitution of the deep lymphatic system. [19] Thus, transfer of healthy tissues may be a means of replacing or re-routing damaged lymphatic vessels to treat or prevent lymphedema. [18] In this study, we demonstrated the lymphatic drainage of the skin flap in 17 patients who presented objective and/or subjective symptoms of lymphedema at the level of the reconstructed breast and/or at the level of the ipsilateral upper limb. These patients had undergone mastectomy with sentinel node (SN) or axillary lymph node dissection (ALND) and immediate or delayed breast reconstruction. Our results suggest firstly that the lymphatic drainage to the ipsilateral axilla is independent of the axillary surgery (in 3/4 patients who had undergone SN biopsy, and 9/13 who had undergone ALND). The restoration of the lymphatic drainage of the “small” areolar skin flap also seems better than for the “larger” skin flap, with significantly (p < 0.05 using Fisher test) higher lymphatic drainage towards the axillary and/or parasternal ipsilateral basins in patients with injection in the areolar flap (12/16) than in those with the injection in the median skin flap (7/18).
Our results (11 patients) can be compared (although not from a statistical point of view because of the small sample size) with those by Matsubara et al. [4] who performed lymphoscintigraphic evaluations of the drainage of the chest wall skin in 24 patients who had undergone a mastectomy with completion ALND but without reconstruction. While they observed no definite change in the drainage after irradiation, they observed drainage toward the ipsilateral axillary lymph node in 6/25 (24%) patients who did not undergo irradiation. The drainage was toward the ipsilateral parasternal lymph nodes in 23/25 (92%), while in our series we observed drainage toward the ipsilateral axillary lymph node in 5/11 (7/13) and toward the ipsilateral parasternal lymph node in 4/11 (6/13). It might be hypothesized that the lower frequency of lymphatic drainage towards the ipsilateral parasternal lymph node in our series (corresponding to one deep drainage) compared with that observed in Matsubara et al. might be related to reconstruction with the interposition of the DIEAP tissues on the deep drainage and/or to the arterial and venous anastomosis of the flap to the corresponding parasternal vessels. Supporting this hypothesis is that the drainage towards the contralateral axillary nodes—depending on the superficial lymphatic drainage—is similar in our series (4/11) and in Matsubara’s (8/25–10/24) while the frequency of contralateral parasternal lymph nodes is lower in our series (1/11) than in Matsubara’s (4/25–8/24).
Our study has several limitations. The study is retrospective, and our sample size was limited. Our observations will need to be validated in a future prospective study including a larger group of patients.
Conclusion
Our results suggest a decrease in deep lymphatic drainage of the skin flap in patients after reconstruction with a DIEAP flap. These results might have implications in the management of patients especially if oedema is observed at the level of the transplanted skin.
Conflict of Interest
All authors disclose that there was no conflict of interest.
REFERENCES
- Tashiro K, Yamashita S, Koshima I, Miyamoto S. Visualization of accessory lymphatic pathways in secondary upper extremity lymphedema using indocyanine green lymphography. Ann Plast Surg.2017; 79: 393-396.
- Bourgeois P. Scintigraphic investigations of the lymphatic system: the influence of injected volume and quantity of labelled colloidal tracer. J Nucl Med 2007; 48: 693-695.
- Bourgeois P, Leduc O, Belgrado JP, Leduc A. Scintigraphic investigations of the superficial lymphatic system: quantitative differences between intradermal and subcutaneous injection. Nucl Med Commun 2009; 30: 270-274.
- Matsubara S, Umehara I, Shibuya H, Okuyama T, Horiushi J, Suzuki S, et al. Radionuclide lymphoscintigraphy performed on the mastectomized chest wall. Cancer 1986; 58: 1225-1230.
- Lawenda BD, Mondry TE, Johnstone PA. Lymphedema:A primer on the identification and management of a chronic condition in oncologic treatment.CA Cancer J Clin 2009;59:8-24.
- Petrek JA, Pressman PI, Smith RA. Lymphedema: Currentissues in research and management. CA Cancer J Clin;2000; 50: 292-307.
- McLaughlin SA, Wright MJ, Morris KT, Giron GL, Sampson MR, Brockway JP, et al. (2008) Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol,2008; 26:5213-5219.
- Lee BB, Laredo J. Contemporary role of lymphoscintigraphy: We can no longer afford to ignore!.Phlebology 2011;26:177-178.
- Hassanein AH, Maclellan RA, Grant FD, Greene AK. Diagnostic accuracy of lymphoscintigraphy for lymphedema and analysis of false-negative test. PlastReconstrSurg Glob Open;2017; 5: 1396.
- Keeley V. The use of lymphoscintigraphy in the management of chronic oedema. J Lymphoedema 2006;1:42-57.
- Szuba A, Shin WS, Strauss HW, Rockson S. The third circulation: radionuclide lymphoscintigraphy in the evaluation of lymphedema. J Nucl Med. 2003;44:43-57.
- Verbelen H, Gebruers N, Beyers T, De Monie AC, Tjalma W. Breast edema in breast cancer patients following breast-conserving surgery and radiotherapy : a systematic review. Breast Cancer Res Treat 2014;147:463-471.
- Cheng MH, Huang JJ, Wu CW, Yang CY, Lin CY, Henry SL, et al. The mechanism of vascularized lymph node transfer for lymphedema: natural lymphaticovenous drainage: PlastReconstrSurg 2014; 133:192-198.
- PB methodological protocol for the scintigrahic investigations of the superficial lymphatic system in patients with limb edemas. 3/05/2017.
- McAllister P, Teo I, Chin K, Makubate B, Munnoch DA. Bilateral breast reconstruction with abdominal free flaps: a single centre, single surgeon retrospective review of 55 consecutive patients. PlastSurgInt 2016; 2016: 6085624.
- Hummelink S, Schultze LJ, Ulrich DJ. Displaying inguinal lymp nodes before transplantation in e deep inferior epigastric perforator flap breast reconstruction using an innovative projection method. J PlastReconstrAesthetSurg 2016; 69:376-380.
- Felmerer G, Muehlberger T, Berens von Rautenfeld D, Vogt PM. The lymphatic system of the deep inferior epigastric artery perforator flap: an anatomical study. British J Plastic Surgery 2002; 55: 335-339.
- Alan Y, Avraham T, Zampell JC, Aschen SZ, Mehrara BJ. Mechanism of lymphatic regeneration after tissue transfer. Plos One 2011; 6: e17201.
- Tamela T, Saaristo A, Holopainen T, Lyytikka J, Kotronen A, Pitkonen M et al. Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nat Med 2007; 13: 1458-1466.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.