Mosaic Trisomy 16 in a Preterm Newborn with Intrauterine Growth Restriction, Hypoglycemia and Atrial Tachycardia
2 Department of Neonatology, American University of Beirut Medical Center (AUBMC), Beirut, Lebanon
3 Pediatric Electrophysiologist Specialist, American University of Beirut Medical Center (AUBMC), Beirut, Lebanon
Received: 02-Sep-2024, Manuscript No. amhsr-24-147137; Editor assigned: 04-Sep-2024, Pre QC No. amhsr-24-147137 (PQ); Reviewed: 18-Sep-2024 QC No. amhsr-24-147137; Revised: 25-Sep-2024, Manuscript No. amhsr-24-147137 (R); Published: 02-Oct-2024
Citation: Lea Chokr. Mosaic Trisomy 16 in a Preterm Newborn with Intrauterine Growth Restriction, Hypoglycemia and Atrial Tachycardia. Ann Med Health Sci Res. 2024; S3: 1-5
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Trisomy 16, responsible for 1%-2% of first trimester losses, poses diagnostic dilemmas in reproductive medicine. While total trisomy 16 is typically lethal, mosaic trisomy 16 cases exhibit diverse phenotypes, often featuring intrauterine growth retardation and cardiac anomalies. Postnatal survival with high levels of mosaic trisomy 16 is rare, with most affected pregnancies ending in spontaneous abortion between 8 and 15 weeks of gestation. However, recent data suggest that select prenatally diagnosed mosaic trisomy 16 patients manifest a mild phenotype and favorable outcomes. Confined placental mosaicism, commonly detected during chorionic villus sampling, emphasizes the complicated nature of this condition. We present a case of mosaic trisomy 16 in a neonate with atrial tachycardia, emphasizing the complexities and treatment strategies in pediatric care. Notably, our management approach involved the novel use of ivabradine alongside propranolol. This case highlights the imperative for ongoing research and collaborative efforts to deepen our understanding and tailor care for the diverse clinical spectrum of mosaic trisomy 16, ensuring comprehensive monitoring and support for affected individuals.
Keywords
Trisomy; Mosaic; Placenta; Intrauterine
Introduction
Trisomy 16 is responsible for 1%-2% of all first trimester losses, making it a challenging diagnosis in reproductive medicine[1]. Although total trisomy 16 is fatal, viable cases of mosaic trisomy 16 have been widely documented in literature. Mosaic trisomy 16 is caused by the postzygotic reduction of one chromosome 16, which salvages a section of the trisomic embryo and/or placenta[1].
Mosaic trisomy 16 newborns have varying phenotypes, which frequently include Intrauterine Growth Retardation (IUGR) and congenital cardiac abnormalities, shedding light on this genetic anomaly[2]. Interestingly, postnatal survival is incompatible with a high level of mosaic trisomy 16, with most trisomy 16 pregnancies spontaneously aborting between 8 and 15 weeks of gestation[3].
Nevertheless, recent data imply that a considerable number of prenatally diagnosed patients with mosaic trisomy 16 have a modest phenotype and a favourable prognosis[4]. Besides, confined placental mosaicism is frequently the cause of mosaic trisomy 16 that is discovered at Chorionic Villus Sampling (CVS). Although a variety of anomalies have been documented, the long-term result in the majority of surviving instances appears to be satisfactory[3]. We hereby present a compelling case report detailing the manifestation of mosaic trisomy 16 coupled with atrial tachycardia, shedding light on its treatment in pediatric population.
Case Presentation
This is a case of a 1860 g male Singleton infant born at 36 weeks and 5 days of gestational age to a 34 year old mother G6P12A3L3. Noninvasive prenatal testing was inconclusive, this along with the previous abortions warranted the obstetrician to do more investigations. Chorionic Villous Sampling (CVS) showed trisomy 16 and Amniocentesis showed 5% mosaic form. On fetal morphology scan, no abnormalities were visualized other than possible intrauterine growth restriction. Urgent delivery was prompted by non-reassuring fetal heart tracing with 6 minutes of bradycardia. Maternal history was significant for autoimmune thyroiditis during a previous pregnancy, osteopenia and polycystic ovarian syndrome.
The infant was born vigorous and required minimal resuscitation efforts, Apgar scores at 5 and 10 minutes were 7 and 9, respectively, with a delivery room temperature of 36.5°C. He was transferred to the Neonatal Intensive Care Unit (NICU) on room air for prematurity, IUGR and further investigation of this mosaicism. Pediatric cardiology consultation on day of life 1 revealed a ventricular septal defect, moderate patent ductus arteriosus, small patent foramen ovale and mild tricuspid regurgitation. Simultaneously, pediatric neurology recommended doing karyotype (which turned out to be normal later on from a blood specimen which was expected) and follow-up as developmental outcomes might be affected later on. Meanwhile, baby was started on IV dextrose and PO feeding due to hypoglycemia, marking the beginning of an obscure glucose management journey. The glucose infusion rate was adjusted keeping point of care glucose levels above 60 mg/dl.
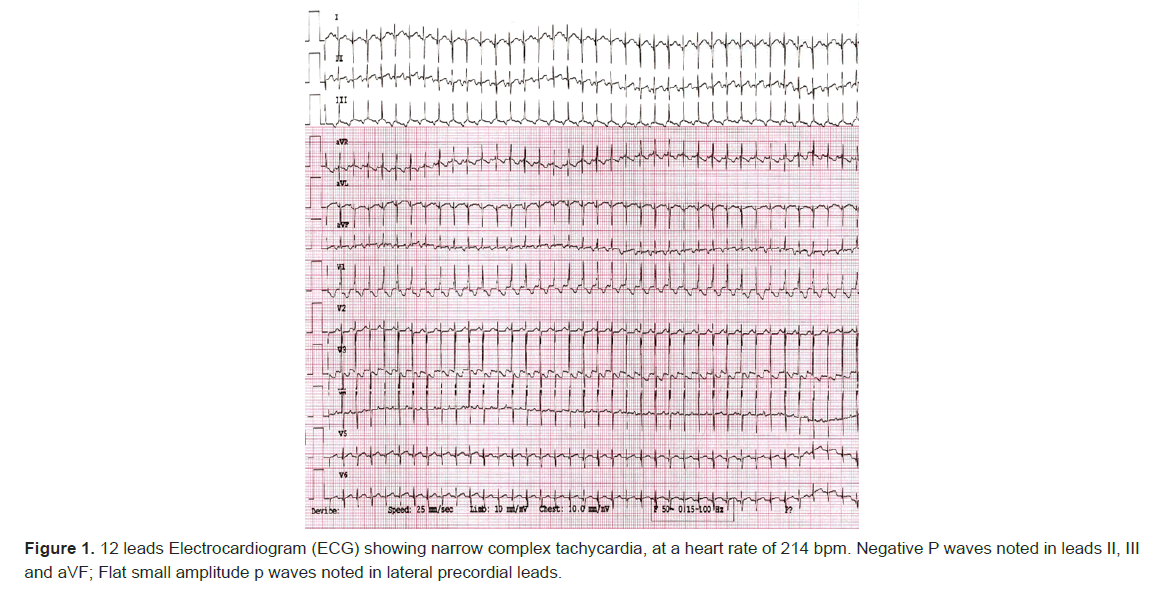
As the neonatal course continued, the focus shifted to treating his new emerging narrow complex tachycardia which was detected on day of life 7 with a heart rate of 230 bpm. Patient started to have frequent paroxysms of tachycardia, that became almost incessant over the next few hours. 12 leads Electrocardiogram (ECG) showed narrow complex tachycardia with negative p waves in leads II, III and augmented vector Foot (avF) (Figures 1,2).
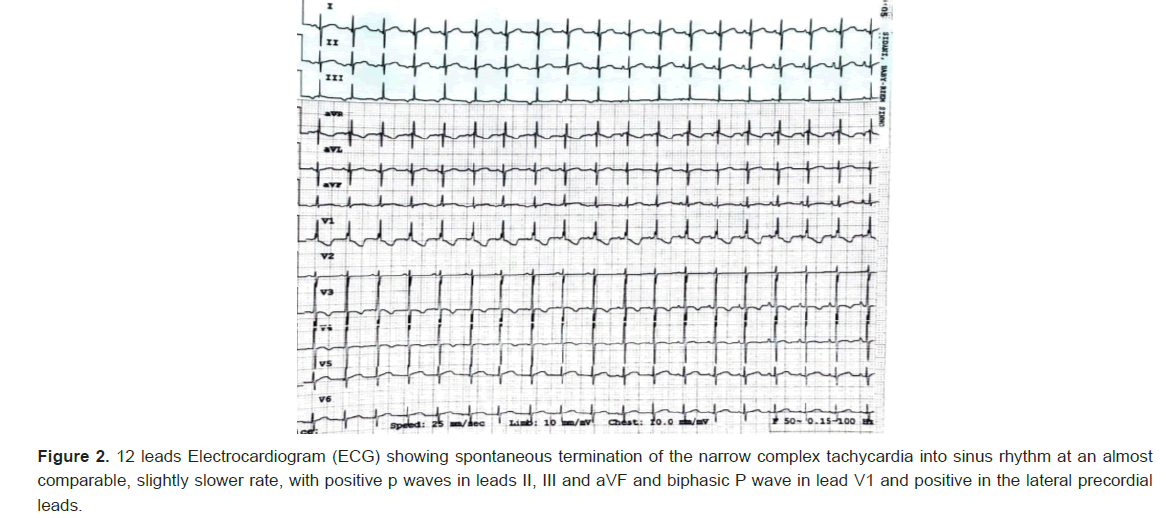
Few intermittent spontaneous terminations to sinus rhythm was noted, defined as normal axis p waves, being positive in inferior leads and biphasic in lead V1. Adenosine was given for diagnostic purposes, however the result was inconclusive, although given 3 times with escalating doses, as termination of the tachycardia occurred every time the saline flush is given following the adenosine push, before the adenosine even starts to act systemically.
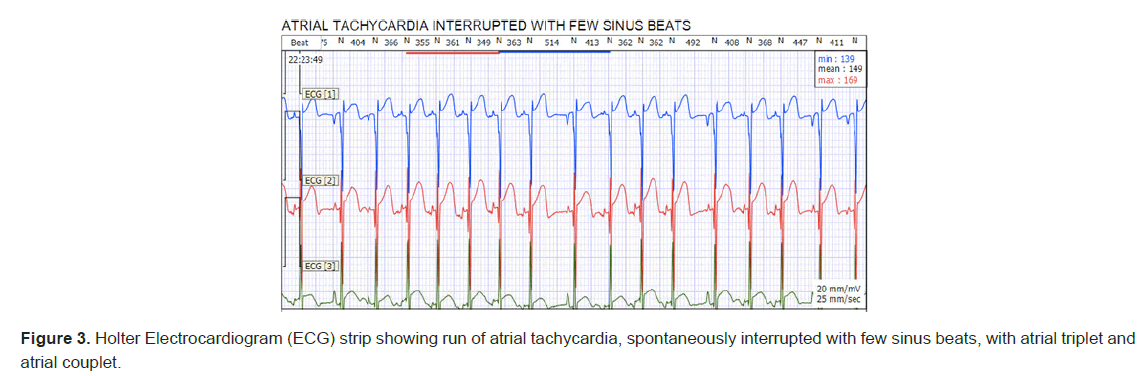
Further rhythm evaluation on telemetry and Holter monitoring revealed findings of automatic atrial tachycardia with spontaneous onset without an apparent critical coupling interval and his episodes showed a cool down behavior before going to sinus rhythm. Frequent PACs and runs of atrial tachycardia were noted his tachycardia was almost in an incessant form on his Holter monitoring (Figure 3). His average HR was 164 bpm, with a maximum heart rate of 230 bpm. Laboratory investigations were done to rule out any other causes of tachycardia. His White Blood Cells (WBC) was benign, at 11200, hemoglobin of 16.5 and platelets count of 220000, normal electrolyte levels and normal Thyroid-Stimulating Hormone (TSH) and C-Reactive Protein (CRP).
Given the automatic nature of his ectopic atrial tachycardia and in view of his underlying hypoglycemia, we opted to start the patient on ivabradine, rather than beta blocker as a first line treatment. The starting dose was 0.05 mg/kg/dose PO every 12 hours. Impressively, the rhythm converted to sinus starting 2 hours post first dose administration. However, more atrial tachycardia runs were noted few hours prior to the next scheduled dose of ivabradine, so the dose was escalated gradually to 0.14 mg/kg/dose every 12 hours over the next couple of days to achieve appropriate rhythm control. Few runs of rate controlled tachycardia were still noted, with a rate similar to his sinus rhythm. QT measurements were normal even after dose adjustments. The patient achieved normoglycemia at day of life 11 with full PO feeding and without IV dextrose support.
The patient underwent circumcision as planned prior to his discharge, however soon he started to develop more frequent episodes of atrial tachycardia, while on ivabradine, that prompted the change of ivabradine schedule to 0.1 mg/kg/dose every 8 hours, with no major change in the frequency of his episodes. Therefore, oral propranolol was introduced as an addon therapy, at 0.1 mg/kg/dose every 12 hours and escalated to 0.3 mg/kg/dose successfully achieving appropriate rhythm control of his tachycardia. This intervention successfully controlled the cardiac rhythm and rate, leading to the patient's discharge with prescribed medications and scheduled close follow-up appointments after a few days.
Discussion
Mosaic trisomy 16 is a rare chromosomal abnormality occurring during embryonic development, leading to a mosaic pattern of both normal and abnormal cells and potentially causing diverse health issues and developmental abnormalities, including cardiac anomalies, intrauterine growth restriction and organ system anomalies[5]. Katherine Neiswanger et al reported 5 cases of mosaic trisomy 16. They found that intra-uterine growth restriction and heart defects commonly occur, even if the mosaicism is thought to be confined to the placenta[1]. These two findings were present in this case.
Rieubland C et al., describes two cases of trisomy 16 mosaicism ascertained postnatally. It was found that intra-uterine growth restriction, abnormal pigmentation of skin, asymmetric craniofacial and body findings, hearing loss, hypospadias, scoliosis and congenital heart defect, are all clinical features described on several occasions in mosaic trisomy 16 diagnosed pre and postnatally[3]. Of all these features, this patient had intrauterine growth restriction, neonatal hypoglycemia and atrial tachycardia.
Atrial tachycardia, as a cardiac finding, has never been described in mosaic trisomy 16 babies so far. This is the first case to report such potential link between mosaic trisomy 16 and such cardiac conduction system abnormalities. Atrial tachycardias often occur in neonates and they have certain features in common. As the atrium is depolarized in an abnormal manner, the P wave has a different shape and/or axis from sinus rhythm. With all forms, the relation of the P wave to the QRS complex may be variable, depending on AV nodal conduction and the ventricular rate can be somewhat irregular if there is variable AV block. Management can usefully be directed at either terminating the tachycardia
In this case, we are dealing with a single focus of ectopic atrial tachycardia, defined as a single abnormal p wave morphology preceding the QRS complexes in tachycardia. These tend to accelerate (“warm up”) and decelerate or stop periodically (“cool down”), but they tend to be incessant at rates around 180–200 beats/min such that they may not present with overt paroxysms of tachycardia, but rather with gradual onset cardiac failure and even dilated cardiomyopathy. This patient fortunately had preserved cardiac function, as the tachycardia was caught up early in his course and addressed appropriately[6].
Options for medical treatment for neonatal ectopic tachycardia include beta blockers, often used in combination with Flecainide, a Ic Anti-arrhythmic medication, in order to achieve rhythm control. Recently, there has been an emerging use of ivabradine in pediatric patients, not only for congenital Junctional Ectopic Tachycardia (JET) and post-operative JET, but even to include focal atrial tachycardia in patients with Congenital Heart Diseases (CHD)[7-9].
Ivabradine is an anti-arrhythmic agent that reduces the heart rate through inhibition of the pacemaker current of the sinoatrial node. It has been used to treat both inappropriate sinus tachycardia and sinus tachycardia related to heart failure in children and adults. Given his high selectivity and minimal side effects, it has been used in pediatric patients with focal atrial tachycardia. Recently, Tolani et al., have reported their early experience of ivabradine use in 15 patients with congenital heart disease presenting with focal atrial tachycardia. The median age at ivabradine initiation was 7 (IQR:1-18) months, with 6 patients (40%) being younger than 3 months old. 80% had complex CHD. Four patients (27%) exhibited diminished ventricular function. Seven patients (47%) developed FAT early after cardiac surgery or interventional catheterization. Ivabradine was used as first-line therapy in 5 patients (33%). 11 patients (73%) received ivabradine in combination with other antiarrhythmic medications. Ivabradine was successful in 80% of the cases, showing a higher response than previously reported for children (50%) and adults without CHD (64%)[7].
The presence of alternative mechanisms of Focal Atrial Tachycardia (FAT), such as micro reentry and triggered activity, might contribute to the insensitivity to ivabradine of some patients[7]. Careful review of telemetry and administration of adenosine could help in diagnosing the mechanism of FAT, thereby optimizing the use of ivabradine for FAT. Back to our case, the use of adenosine was not helpful, as the patient was showing spontaneous tachycardia termination. However, we were able to identify the mechanism of the atrial tachycardia as being automatic given its gradual warm up and cool down behavior through careful rhythm evaluation.
Ivabradine was considered as first line treatment in our case instead of beta blockers given the ongoing hypoglycemia that the patient was experiencing. Moreover, we tried to avoid the use of flecainide in such young population as we don’t have the availability at our facility to measure its plasma level in order to perform appropriate dose titration given its narrow therapeutic index.
In contrast, ivabradine use in our patient, who is among the youngest to receive it, has been shown to be safe, with no reported side effects noted, even when used in combination with other anti-arrhythmic medications such as beta blockers. Common side effects of ivabradine include bradycardia, phosphenes, blood pressure changes, increased risk of atrial fibrillation, gastrointestinal symptoms, headache, sleep disturbances and allergic reactions, none of which were observed in the patient[10].
Intrauterine growth restriction is another common finding in individuals with mosaic trisomy 16. Restricted growth in mosaic trisomy 16 is linked to placental insufficiency, resulting from abnormalities in placental structure and function, disrupting normal development and causing inadequate nutrient and oxygen supply to the fetus, contributing to intrauterine growth restriction. A meta-analysis done by Spinillo et al., suggests that pregnant women prenatally diagnosed with confined placental mosaicism have an increased risk of impaired fetal growth, suggesting the need for intensified antenatal surveillance[11]. This comes in parallel with our patient, born with intra-uterine growth restriction.
The presence of atrial tachycardia and intrauterine growth restriction in an individual with mosaic trisomy 16 should prompt close monitoring and multidisciplinary care involving pediatric cardiology, pediatric electrophysiology, fetal medicine and genetic specialties. The management of this condition requires a comprehensive approach, including regular monitoring of cardiac function, growth assessment and appropriate interventions to address potential complications.
Langlois S et al., in postnatal follow-up of prenatally diagnosed trisomy 16 mosaicism, found that the majority of prenatally diagnosed trisomy 16 mosaic cases have a good postnatal outcome. However, the finding of mosaicism on amniotic fluid and the presence of major congenital anomalies are associated with an increased risk of developmental delay[4]. In our case, the patient did not have major congenital anomalies, however postnatal follow up and developmental screening should be adopted. In a study done by Sparks et al., it was found that the majority of children affected by mosaic trisomy 16 or confined placental mosaicism, demonstrate normal neurodevelopmental outcomes and high health related quality of life[12]. As such, our patient will be followed up to assess him neurodevelopmentally and globally.
It's important to note that mosaic trisomy 16 can have highly variable clinical presentations and the specific manifestations can vary among affected individuals. A review done by Peter Benn on trisomy 16 and trisomy 16 mosaicism, suggests that there are diverse outcomes for trisomy 16 infants, including fetal death, prematurity, low birth weight, isolated birth defects and more complex multiple congenital anomalies. Analysis using FISH of the specific tissues involved in abnormalities may also provide additional insights[2]. As such, it would be interesting to analyze the patient’s abnormal tissues, especially cardiac, using FISH. The severity and prognosis of mosaic trisomy 16 depend on the extent and distribution of abnormal cells in the body and the associated organ system involvement. Genetic counseling and ongoing medical follow-ups are crucial for individuals with mosaic trisomy 16 to address their specific needs and optimize their overall health and well-being. Lastly, Chen CP et al., found that the abnormal trisomy 16 cell line in mosaic trisomy 16 may disappear after birth[13]. This is worth following up with our patient later in his life.
Conclusion
In conclusion, this case report provides a glimpse into the complexities of mosaic trisomy 16, showcasing a neonatal presentation marked by atrial tachycardia. The management, involving medications like ivabradine and propranolol, reflects the delicate balance required in addressing associated challenges, including neonatal hypoglycemia. The case highlights the need for ongoing research and collaborative efforts to better understand and navigate the diverse clinical spectrum of mosaic trisomy 16, ensuring tailored care and long-term monitoring for affected individuals.
References
- Neiswanger K, Hohler PM, Hively‐Thomas LB, McPherson EW, Hogge WA, et al. Variable outcomes in mosaic trisomy 16: Five case reports and literature analysis. Prenat Diagn. 2006; 26(5):454-461.
- Benn P. Trisomy 16 and trisomy 16 mosaicism: A review. Am J Med Genet. 1998; 79(2):121-133.
- Rieubland C, Francis D, Houben L, Corrie S, Bankier A, et al. Two cases of trisomy 16 mosaicism ascertained postnatally. Am J Med Genet A. 2009; 149(7):1523-1528.
- Langlois S, Yong PJ, Yong SL, Barrett I, Kalousek DK, et al. Postnatal follow‐up of prenatally diagnosed trisomy 16 mosaicism. Prenat Diagn. 2006; 26(6):548-558.
- Nguyen HH, Umapathi KK, Bokowski JW, Hogan K, Hart A, et al. Mosaic Trisomy 16 Associated with Left Lung Agenesis, Abnormal Left Arm, and Right Pulmonary Artery Stenosis: Expanding the Phenotype and Review of the Literature. J Pediatr Genet. 2022; 11(04):324-332.
- Kothari DS, Skinner JR. Neonatal tachycardias: An update. Arch Dis Child Fetal Neonatal Ed. 2006; 91(2):F136.
- Tolani D, Ramdat Misier NL, Alqahtani M, Tindel K, Scott WA, et al. Early experience with ivabradine for focal atrial tachycardia in pediatric patients with congenital heart disease. Heart Rhythm. 2023: 2023-2006.
- AL‐GHAMDI SA, AL‐FAYYADH MI, Hamilton RM. Potential new indication for ivabradine: Treatment of a patient with congenital junctional ectopic tachycardia. J Cardiovasc Electrophysiol. 2013; 24(7):822-824.
- Krishna MR, Kunde MF, Kumar RK, Balaji S. Ivabradine in post-operative junctional ectopic tachycardia (JET): Breaking new ground. Pediatr Cardiol. 2019; 40:1284-1288.
- Buda V, Prelipcean A, Cozma D, Man DE, Negres S, et al. An up-to-date article regarding particularities of drug treatment in patients with chronic heart failure. J Clin Med. 2022; 11(7):2020.
- Spinillo SL, Farina A, Sotiriadis A, Pozzoni M, Giglio S, et al. Pregnancy outcome of confined placental mosaicism: Meta-analysis of cohort studies. Am J Obstet Gynecol. 2022; 227(5):714-727.
- Sparks TN, Thao K, Norton ME. Mosaic trisomy 16: What are the obstetric and long-term childhood outcomes? Genet Med. 2017; 19(10):1164-1170.
- Chen CP, Lan FH, Chern SR, Wu PS, Chen SW, et al. Prenatal diagnosis of mosaic trisomy 16 by amniocentesis in a pregnancy associated with abnormal first-trimester screening result (low PAPP-A and low PlGF), intrauterine growth restriction and a favorable outcome. Taiwan J Obstet Gynecol. 2021; 60(6):1107-1111.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.