Pattern of CD4 T‑lymphocyte Values in Cancer Patients on Cytotoxic Therapy
- *Corresponding Author:
- Dr. Anazoeze Jude Madu
Department of Hematology and Immunology, University of Nigeria, Enugu Campus, Enugu, Nigeria.
E-mail: anazoeze@yahoo.com
Abstract
Background: Assessment of patients prior to cytotoxic chemotherapy usually includes absolute neutrophils count. Other cellular markers of susceptibility to infection as well as immunocompetence include the T Helper lymphocyte count. In cancer patients, decrease in these lymphocytes has been observed to be associated with decreased overall survival. Aim: To assess the degree of CD4 lymphopenia encountered during cytotoxic chemotherapeutic treatment for cancer and evaluate the differences observed for the various drug combinations. Subjects and Methods: Eighty patients with various histologically diagnosed malignancies had their CD4 lymphocyte counts carried out at days 0 and 12 of the first cycle of their various chemotherapeutic regimens. They were adult patients who had been diagnosed with breast cancer 36/80 cases (45%), non‑Hodgkin’s lymphoma 8/80 cases (10%), Hodgkin’s lymphoma 13/80 cases (16.3%), multiple myeloma 7/80 cases (8.8%), colorectal carcinoma 6/80 cases (7.5%), and other malignancies 10/80 cases (12.5%). CD4 lymphocyte count was done using the Partec Cyflow® 2000 CD4 cell counter, and their socio‑demographic data of the patients were assessed using a questionnaire. Results: The mean (sd) CD4 lymphocyte count pre‑ and post‑chemotherapy was observed to be 567 (341) cells/μLand 349 (207) cells/μL while the median values were 454 cells/μLand 349 cells/μL respectively. There were significant differences in CD4 lymphocyte counts after chemotherapy compared to the pre‑chemotherapy values. Conclusion: Epirubicin combinations used in breast cancer patients as well as (Adriamycin, Bleomycin, Vinblastine, Dacarbazine) ABVD regimen used in treatment of Hodgkin’s lymphoma were found to be significantly less lymphotoxic than other chemotherapeutic combinations. These drugs or their combinations may be less immunotoxic than other known regimen used for these malignancies.
Keywords
Cancers, CD4 lymphocyte, Cytotoxic chemotherapy, Immunosuppression
Introduction
Cytotoxic chemotherapy as well as malignancies is associated with an increased risk of immunosuppression and occurrence of infections. The tendency to develop infection as well as its severity increases with higher dosage and longer duration of chemotherapy.[1] Many factors increase the susceptibility of cancer patients to immunosuppression and subsequent infection.[1,2] It is a well-known fact that most anti-cancer drugs induce myelosuppression and that this in effect leads to depletion of both cellular and humoral immune system. Destruction of all the rapidly proliferating cells, which include the myeloid and lymphoid cells also occurs pre-disposing to infection.[3]
Activation of T-cells occur after recognition of specific antigens through cell surface receptors and results in replication and or mediation of one of three functions; cytotoxicity, by direct killing of specific target cells; helper function, by stimulating the immune response of the other cells; and suppressor function, by inhibiting the immune response of other cells.[4,5] Helper T-cells are mainly cytokine secreting cells. They can be subdivided in to two major types: Type 1 (Th1), which secrete interleukin (IL)-2, and interferon- γ and type 2 (Th2), which secrete IL-4, IL-5, IL-6, and IL-10.[4,5] However, T-lymphocyte have an impact on practically all aspects of the immune system as a consequence of their ability to induce specific immune response in other cells.[1,4,5]
Normal reference value for CD4 +ve T-lymphocyte count in the Caucasian population is 400-1500 cells/μL while a mean (sd) absolute CD4 count of 665.6 (246.8) within 95% confidence interval (CI) of 588.7-742.5 cells/μL was obtained in a Tanzanian study of which majority were blacks.[6] However, studies carried out at Jos University Teaching Hospital reported a mean CD4 +ve T-lymphocyte count of 830 cells/μL, with a range of 514-1207 cells/μL, in healthy Nigerian adults.[7] It is known that some malignancies as well as their treatment cause marked and sustained depletion of CD4 +ve T-lymphocyte count.[6] Previous reports in human immunodeficiency virus (HIV) infection have shown that susceptibility to opportunistic infection increases dramatically with CD4 +ve T-cell counts less than 200 cells/μL.[8] It then follows that any condition which depletes the CD4 +ve T-cell count appreciably will also render these patients susceptible to these infections.
Lymphopenia as well as CD4 lymphocyte depletion also occurs alongside neutropenia in cancer patients and have been noted in some studies as a predictor of early death in patients on chemotherapy.[2] The occurrence of immunosuppression and thus infection in cancer patients presents additional challenges in management and care of these patients. Some cytotoxic combinations may have a more deleterious effect in this regard than others. Standard chemotherapy regimen may need to be altered in dosage or duration to avoid these deleterious outcomes. Prophylactic or therapeutic antibiotics may be required in addition to antifungal and antiviral agents.
This central role played by the CD4 lymphocytes in mounting an immune response should not be neglected. It is therefore necessary to assess the immune status of every individual before and during each course of chemotherapy as both granulocyte and lymphocytes contribute to immuno-competence. This study aims to assess the changes in CD4 lymphocyte counts occurring in cancer patients as a possible modality of evaluating a more potent cell lineage involved in both cellular and humoral immune response.
Subjects and Methods
The study was conducted on cancer patients seen at the University of Nigeria Teaching Hospital, Enugu, Nigeria. This is a tertiary health institution and a referral center located in the Southeastern part of Nigeria. Patients, who gave informed consent, were recruited from the Oncology clinic of the hospital, which operates a joint clinic run by the general surgery, hematology/oncology, gynecology as well as other medical and surgical specialties involved in cancer care and treatment. Ethical clearance was obtained from the hospital Ethical Review Committee.
Eighty adult patients who had been histologically diagnosed with malignancy (which is usually treated with cytotoxic chemotherapy) and had not received any form of cytotoxic chemotherapy were recruited consecutively from the out-patient department and wards. For each patient who was chemotherapy–naïve and gave written informed consent, 3 mlofanti-coagulated venous blood was collected prior to chemotherapy and on the 12th day of the first cycle of cytotoxic treatment. Patients who had diagnosed concurrent infections of any type or who had received any form of cytotoxic agents in the past were exempted. Their CD4 lymphocyte estimation was carried out using flow cytometry (Partec Cyflow® Automatic CD4 cell counter).
Data were processed on desktop computers using Graph Pad Prism 2.0® (United Kingdom, 2009) software package. Inferential analysis was carried out using a non-parametric t-test (Mann-Whitney test of significance) as the data collected had a non-Gaussian distribution. The transformed data was then used for the calculation of the paired sample t-test for the individual patient groups. Data transformation was performed using the log scale to obtain a normally distributed data set. Statistical significance was set at P< 0.05, with a 95%CI.
Results
The patient’s ages ranged from 18 years to 80 years, with a median age of 45 years. There were 27/80 males (33.7%) and 53/80 females (66.2%). There were 4 patients in the 18-20 year age group, 8 patients aged 21-30 years, 18 patients aged 31-40 years, 24 patients aged 41-50 years, 11 patients aged 51-60 years, 11 patients aged 61-70 years, and 4 patients were over 70 years of age. The two most common malignancies, which together accounted for more than 60% (48/80) of the cases were breast cancer 36/80 cases (45%), and non-Hodgkin’s lymphoma (NHL) 16.2% (13/80), forty one (51.3% - 41/80) of the patients presented in the early stage of their malignancies while 39/80 (48.7%) of them presented with advanced cancers.
In all 36/80 (45%) cases of breast cancer were seen and these patient’s ages ranged from 26 years to 69 years with a mean age of 47.58 years and this consisted of 97% (35/36) females and 3% (1/36) male patient. Most of the patients (58.3%-21/36) presented with advanced disease while 15/36 (41.7%) of them presented with the early stage of the disease. Their mean CD4 counts pre-and post-chemotherapy are shown in Table 1.
A total of 8/80 (10%) of the patients had NHL, 6/8 (75%) of these were males while 2/8 (25%) were females and their ages ranged from 18 to 67 and a mean age of 39.13 years. A total of 6/8 (75%) of the patients presented with the early stages of the disease (stages I and II) while 2/8 (25%) presented with the later stages of the disease.
A total of 13/80 (16.2%) of the cases had Hodgkin’s lymphoma, age 18-65 years with a mean age of 37.9 years. Of these 9/13 (69.2%) presented with the early stages of the disease while 4/13 (30.8%) had the advanced stages of the disease.
A total of 7/13 (53.8%) of them were males while 6/13 (46.2%) were females.
In all, 7/80 (8.8%) of the patients had multiple myeloma, of whom 4/7 (57.1%) were males and 3/7 (42.9%) were females. Their ages ranged from 31 years to 75 years, with a median age of 58 years. The majority of patients, 6/7 (85.7%) presented with advanced disease (Durie and Salmon stages III or IIb), while only one patient had early disease.
A total of 6/80 (7.5%) patients in all, 3/6 (50%) males and 3/6 (50%) females, had colorectal carcinoma. Their ages ranged from 32 years to 80 years, with a median age of 46 years, and 4/6 (66.7%) had early disease while 2/6 (33.3%) had late disease.
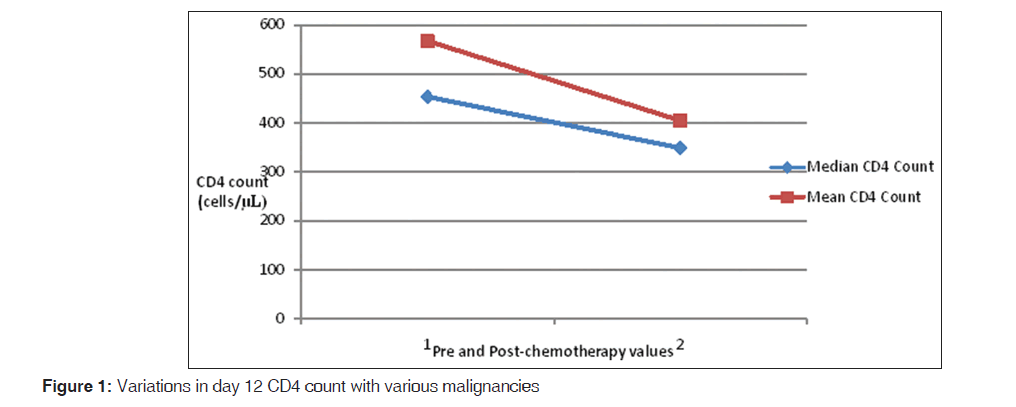
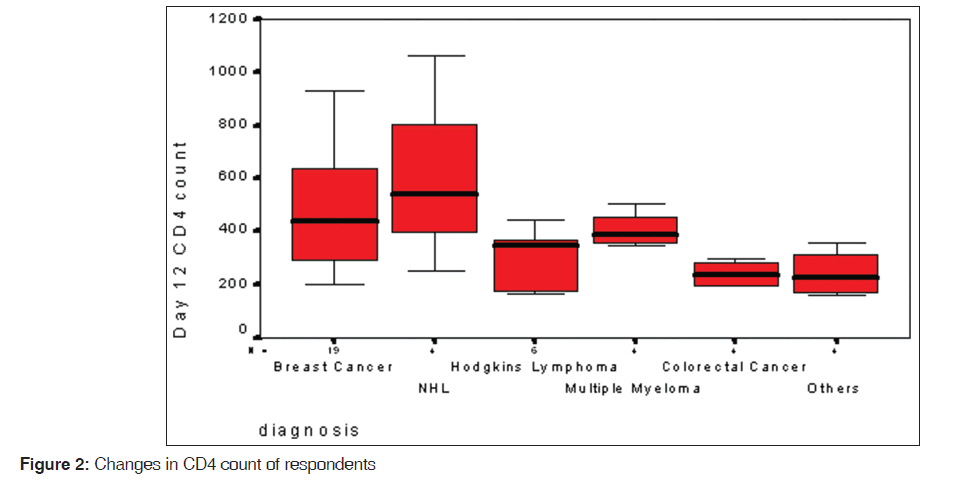
The changes in each individuals patients CD4 count with chemotherapy are shown in Figure 1. The overall mean CD4 lymphocyte count pre-and post-chemotherapy was observed to be 567 (341) cells/μL and 349 (207) cells/μL while the median values were 454 cells/μL and 349 cells/μL respectively. The mean (sd) pre-chemotherapy CD4 lymphocyte count was 534 (326) cells/μL for males and 625 (371) cells/μL for females [Figure 2]. While the mean post-chemotherapy CD4 counts were; 363 (175) cells/μL for males and 436 (243) cells/μL for females.
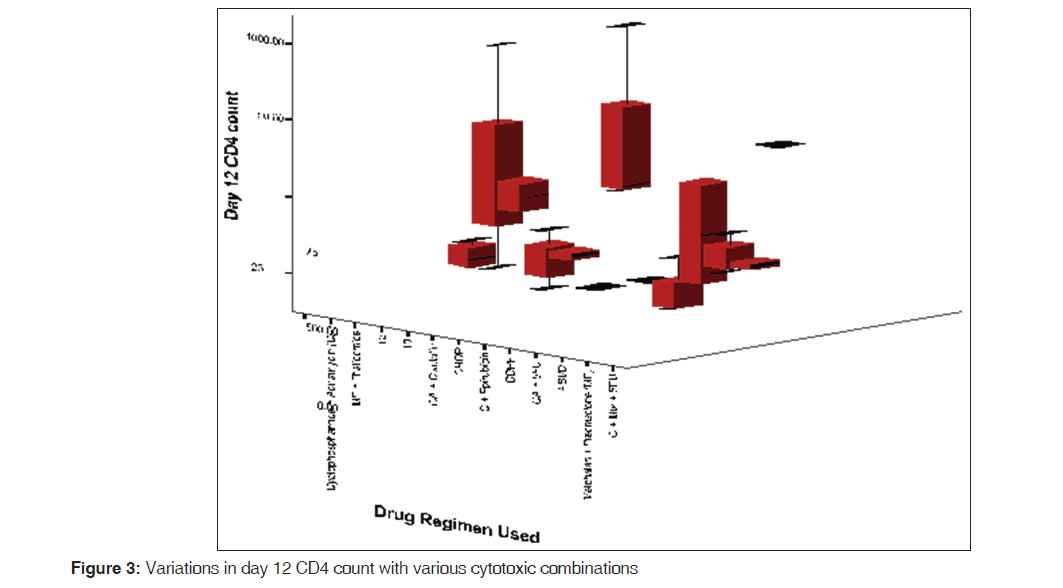
A significant drop in the CD4 lymphocyte count was observed in breast cancer patients (P< 0.001). Majority of the patients in this group received cyclophosphamide and adriamycin, which caused a significant CD4 lymphopenia (P< 0.001), compared to those on cyclophosphamide and epirubicin (P = 0.96). Patients who had NHL were also observed to have significant lymphopenia (P = 0.04), and 75% (6/8) of these patients were given cyclophosphamide, hydroxodaunorubicin, vincristine, and prednisolone (CHOP) regimen. Patients who had Hodgkin’s lymphoma were placed on adriamycin, bleomycin, vinblastine and dacarbazine (ABVD) (53.8% - 7/13) or cyclophosphamide, oncovin, procarbazine and prednisolone (COPP) (46.2% - 6/13). Those on ABVD showed a lower CD4 depletion (P = 0.57) than those on COPP (P = 0.04). The variations in day 12 CD4 lymphocyte counts observed in the various cancer groups are shown in Figure 3 with the various cytotoxic combinations are recorded in Table 2 while the observations in various types of cancers are noted in Table 1.
| Diagnosis | Freq | Parameter (cells/µL) | Day 0 (SD) | Day 12 (SD) | t-test (P value) |
|---|---|---|---|---|---|
| Ca breast | 36 | CD4 | 613 (329) | 428 (239) | 5.782 (0.001)* |
| NHL | 8 | CD4 | 642 (261) | 514 (273) | 2.460 (0.04)* |
| Hodgkin’s lymphoma | 13 | CD4 | 502 (442) | 351 (191) | 1.766 (0.10) |
| Multiple myeloma | 7 | CD4 | 566 (439) | 410 (178) | 0.993 (0.36) |
| Colorectal Ca | 6 | CD4 | 642 (325) | 375 (189) | 2.818 (0.04)* |
| Other cancers | 10 | CD4 | 525 (376) | 324 (180) | 2.635 (0.03)* |
*Statistically Significant, P<0.05, SD: Standard deviation, Freq: Frequency, ANC: Absolute neutrophil count, CD4: CD4 positive lymphocyte count, Ca: Cancer, NHL: Non-Hodgkin’s Lymp
Table 1: Mean values CD4 counts recorded in different malignancies pre- and post-chemotherapy
| Drug regimen | Freq | Day 0 (SD) | Day 12 (SD) | t-test (P value) |
|---|---|---|---|---|
| Cyclo and adriamycin | 30 | 622 (374) | 443 (255) | 5.083 (0.001)* |
| C and A+cisplatin | 4 | 668 (178) | 272 (6) | 3.263 (0.19) |
| CHOP | 6 | 728 (287) | 584 (280) | 1.850 (0.14) |
| C and epirubicin | 3 | 666 (169) | 281 (53) | 2.452 (0.96) |
| COPP | 6 | 378 (190) | 262 (134) | 2.828 (0.04)* |
| C and A+5-FU | 3 | 943 (301) | 623 (42) | 1.751 (0.330) |
| ABVD | 7 | 453 (444) | 352.0 (48) | 0.613 (0.57) |
| M and P | 4 | 536 (151) | 504 (176) | 0.462 (0.69) |
| C, Mtx and 5-FU | 5 | 539 (293) | 468 (408) | 0.877 (0.54) |
| M, P and T | 3 | 949 (756) | 453 (66) | 0.854 (0.55) |
| FAC | 5 | 606 (362) | 305 (89) | 1.915 (0.15) |
| Others | 4 | 528 (375) | 380 (224) | 3.048 (0.001)* |
*Statistically Significant, (P<0.05), SD: Standard deviation, C: Cyclophosphamide, M: Melphalan, P: Prednisolone, Mtx: Methotrexate, Thal: Thalidomide, FAC: 5-FU, Adriamycin, Cisplatin, 5-FU: 5-Fluorouaracil, C: Cyclophosphamide, O: Oncovin (Vincristine), P: Procarbazine, A: Adriamycin, H: Hydroxodaunorubicin, P: Prednisolone, B: Bleomycin, V: Vinblastine, D: Dacarbazine
Table 2: Observed mean CD4 counts with different cytotoxic regimen
Figures 1 and 2 show the variation in CD4 counts for the various malignancies as well as variations in patients on the various cytotoxic drug combinations.
Discussion
The mean (sd) CD4 lymphocyte count recorded before and after chemotherapy in this study was found to be 594 and 412 cells/μL, which was within the normal reference ranges for Nigerians, except the day 12 CD4 lymphocyte count. The change in this parameter from the pre-chemotherapy values was statistically significant. This is similar to the findings of Khan et al. in his study on solid tumors.[9]
Breast cancer
Individuals diagnosed with breast cancer in this study were observed to have a significant depletion in their CD4 lymphocyte values. This is similar to the findings of Hakim et al., although in their study the patients received5-fluoroucil, leucovorin, adriamycin, cytoxan [10] or paclitaxel and evaluation was carried out after the1st month of therapy. It was also observed in this study that adriamycin (hydroxodaunorubicin), when replaced by epirubicin caused less significant CD4 lymphopenia P = 0.96). Larger studies may be required to promote the use of epirubicin over daunorubicin, which causes additional cardiotoxicicty. Non-Hodgkin’s lymphoma
The change in CD4 lymphocyte count in these patients were found to be significant (P = 0.04), most of the patients in this group received CHOP chemotherapy. In the study carried out by Mackall significant depression in CD4 count was observed, however, the NHL patients in this study received higher doses of chemotherapy (cyclophosphamide 1.6 g/m2).[11] Though in these studies a low baseline CD4 count was recorded, this was not supported by our study. The absence of an alternative regimen to CHOP restricted the ability of this study to offer due comparison.
Hodgkin’s lymphoma
Individuals with Hodgkin’s lymphoma in this study had normal baseline mean CD4 counts as observed in previous studies. There was significant depletion in CD4 lymphocyte values in patients who received COPP (P = 0.04) compared to those who received ABVD (P = 0.57). This observation is not supported by any previous study; however, ABVD has been noted to increase overall survival and disease free survival in Hodgkin’s lymphoma patients. Previous reports show that ABVD is less toxic to the gonads, and thus cause less infertility than COPP, findings of this study; however suggests that it may actually be less immunotoxic. Though a larger study may be required to confirm the safety of ABVD over COPP, there will be few indications to use the older regimen as more studies show the superiority of the newer combination.
Multiple myeloma
The mean pre-chemotherapy CD4 count was within normal reference range, this was similar to the finding by Borg’s group, who recorded a median CD4 count of 650 cells/μL.[12] However, in this study melphalan-based combinations were used for the majority of the patients, while in Borg’s study other dexamethasone-based combinations were preferred. Though similar outcomes were recorded in both studies, it must be noted that in Borg’s study some of the patients had been exposed to previous chemotherapeutic treatment and the pre-chemotherapy values were assessed on day 5 of the cycle.
Colorectal carcinoma
Individuals in this group received the most varied collection of chemotherapeutic agents this indicates the varied choices of the managing surgeon. A significant depletion of the CD4 count in these patients were observed, Milasiene et al. had noted that in patients with colorectal cancer a low CD4 lymphocyte count pre-surgery was positively correlated with reduced survival.[13] This buttresses the point that any regimen to be used in this group of patients should have the least toxic effect on the lymphocyte series.
Other malignancies
The pre-chemotherapy mean CD4 count in this group was the second lowest, after Hodgkin’s lymphoma, also the change in this parameter was found to be significant. The individuals in this group had a wide variety of malignancies viz.; osteosarcoma, cervical cancer, liposarcoma, squamous cell carcinoma, histiocytoma, seminomaas well as cancers of the larynx, ovaries, stomach, and urinary bladder. These patients also received a wider variety of drugs compared to other groups; it is therefore not surprising that the change in both parameters were found to be significant in this study as had been observed in others studies.
Depletion of CD4 lymphocytes due to chemotherapy may be such as to approach counts seen in HIV infection and therefore leads to occurrence of opportunistic infections. Significant differences exist in the degree of lymphopenia caused by the various cytotoxic combinations. Larger studies will be required to evaluate the degree of immunosuppression observed with the different agents and less toxic agents may be favored in order to reduce morbidity and mortality.
Conclusion
Chemotherapeutic regimen including cyclophosphamide/ adriamcyin, as well as COPP have been found in this study to cause significant depletion in CD4 lymphocyte of patients. Patients on these combinations will suffer more CD4 lymphopenia and may require prophylactic antiviral and anti-fungal agents, but further studies are required to validate these findings. Findings of this study indicate that cyclophosphamide/epirubicin combination for treatment of breast cancer, as well as ABVD, used in treating Hodgkin’s lymphoma, causes less significant CD4 lymphopenia. Further studies involving larger patient groups may be necessary to consolidate on this finding and also proffer less immunotoxic combinations, in order to reduce hospital stay and improve overall survival.
Limitations of the study
The low number of patients recruited for this study caused an obvious limitation in the interpretation of the findings of this study and its power
Source of Support: We declared that the work was sponsored by the authors and no financial support was received.
Conflict of Interest: None declared.
References
- RolstonKV.BG. Infections in patients with cancer. Cancer Medicine. 6th ed.Ontario, BC: DeckerInc.;2002.p. 355-70.
- Sharma A, Lokeshwar N. Febrile neutropenia in haematological malignancies. J Postgrad Med 2005;51:S42-8.
- O'Brien S N, Blijlevens N M, Mahfouz T H, Anaissie E J. Infections in patients with hematological cancer: Recent developments. Hematology Am Soc Hematol Educ Program 2003:438-72.
- Delves P J, Roitt I M. The immune system. Second of two parts. N Engl J Med 2000;343:108-17.
- Delves P J, Roitt I M. The immune system. First of two parts. N Engl J Med 2000;343:37-49.
- Ngowi B J, Mfinanga S G, Bruun J N, Morkve O. Immunohaematological reference values in human immunodeficiency virus-negative adolescent and adults in rural northern Tanzania. BMC Infect Dis 2009;9:1.
- Njoku M O, Sirisena N D, Idoko J A, Jelpe D. CD4+ T-lymphocyte counts in patients with human immunodeficiency virus type 1 (HIV-1) and healthy population in Jos, Nigeria. Niger Postgrad Med J 2003;10:135-9.
- Osarogiagbon RU. Immunology in cancer. Archives of Ibadan Medicine 2002;1:20-4.
- Khan S, Dhadda A, Fyfe D, Sundar S. Impact of neutropenia on delivering planned chemotherapy for solid tumours. Eur J Cancer Care (Engl) 2008;17:19-25.
- Hakim F T, Cepeda R, Kaimei S, Mackall C L, McAtee N, Zujewski J, et al. Constraints on CD4 recovery postchemotherapy in adults: Thymic insufficiency and apoptotic decline of expanded peripheral CD 4 cells. Blood 1997;90:3789-98.
- Mackall C L. T-cell immunodeficiency following cytotoxic antineoplastic therapy: A review. Stem Cells 2000;18:10-8.
- Borg C, Ray-Coquard I, Philip I, Clapisson G, Bendriss- Vermare N, Menetrier-Caux C, et al. CD 4 lymphopenia as a risk factor for febrile neutropenia and early death after cytotoxic chemotherapy in adult patients with cancer. Cancer 2004;101:2675-80.
- Milasiene V, Stratilatovas E, Norkiene V. The importance of T-lymphocyte subsets on overall survival of colorectal and gastric cancer patients. Medicina (Kaunas) 2007;43:548-54.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.