Periodic Peritoneal Dialysis in End Stage Renal Disease: Is it Still Relevant? A Single Center Study from India
Citation: Gandhi K, Prasad D, Malhotra V, Agrawal D, Beniwal P, Mathur M. Periodic peritoneal dialysis in end stage renal disease: Is it still relevant? A single center study from India. Ann Med Health Sci Res 2015;5:379-84.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: High cost of maintenance hemodialysis (HD) and continuous ambulatory peritoneal dialysis (PD) in India has made renal replacement therapy out of reach of many patients with end stage renal disease (ESRD). Repeated puncture PD although inferior to HD biochemically, is easily and freely available across Rajasthan, India, and is simple to perform, and does not require sophisticated machines, thus making it an attractive option for dialysis for ESRD. Aim: To analyze the outcomes of periodic PD in patients with ESRD requiring dialysis support. Subjects and Methods: A prospective study analyzing the data of patients who underwent PD between August 2010 and January 2013 in Sawai Man Singh Hospital, Jaipur, India was conducted. Patients were divided into three groups based on the time period between first and second session of PD. Detailed demographic and clinical data during the study period were collected along with PD related complications. The main outcome studied was technique survival 1 year post initiation of PD. Results: 234 patients received an initial session of PD, of which 174 had a good response and were included in the study. 19 patients received the second PD within 7 days of first (Group 1), 45 patients within 8–14 days (Group 2) and 110 patients within 15–21 days (Group 3). The overall 1 year technique survival was 68.4% (91/133), with a rate of 50% (5/10), 56.8% (21/37), and 75.6% (65/86) for Group 1, Group 2, and Group 3, respectively. The time duration between first and second PD proved to be reliable indicator of the subsequent response, with a technique survival rate significantly lower in Group 1 patients compared to Groups 2 and 3 (P = 0.04). Median dialysis free days were 11, 16 and 21 days in Group 1, Group 2, and Group 3, respectively. Peritonitis rate observed was 2.1% (49/2261) during the study period. Conclusion: Periodic PD is a simple, safe and cheap procedure, which can be considered as used as a palliative measure in terminal uremia in underprivileged areas.
Keywords
Peritoneal dialysis, End stage renal disease, Renal replacement therapy
Introduction
India has approximately 55,000 patients on dialysis. The crude and age‑adjusted end stage renal disease (ESRD) incidence rates are 151 and 232 per million populations, respectively.[1,2] Most of hemodialysis (HD) centers in India are funded by private firms and perform both transplant‑oriented dialysis as well as maintenance HD (MHD). In comparison, maintaining an MHD program is practically not feasible in government run sectors considering the high cost of operation. Therefore, most of the government run hospitals operate renal transplant‑oriented HD facilities. The cost of twice a week HD comes to Rs. 29,852 (USD 481) per month.[3] Continuous ambulatory peritoneal dialysis (CAPD) is also expensive, with an average monthly cost of Rs. 28,763 (USD 464) for 3 sessions/day.[3] The per capita income in India is approximately USD 640[3], and almost 25–30% of the population is still below poverty line. Therefore, the financial burden posed by renal replacement therapy (RRT) for the poor and their family is huge leading to frequent dropouts and noncompliance. The poor quality of life only adds to the morbidity and mortality. In 2011 an ambitious scheme “Chief Ministers free medicine scheme” was announced in Rajasthan, India, which made essential drugs, including PD fluid and rigid‑trocar type PD catheter freely available to the poor and needy in government hospitals across Rajasthan. Since then, periodic PD using a repeated puncture technique is frequently provided to ESRD patients in our center.
Our aim was to study the outcome of this form of PD in ESRD.
Subjects and Methods
Demographic and clinical data
This was a prospective, observational study including adults with ESRD, who were initiated on repeated puncture PD at Sawai Man Singh Hospital, Jaipur, Rajasthan, India from August 2010 to January 2013. A sample size of consecutive patients, aged >18 years with incident ESRD (estimated glomerular filtration rate [eGFR] <15 ml/ min) presenting to our hospital for the 1st time and those who had never received dialysis previously were included in the study and followed up for 1 year. The main indications for initiation of HD were (a) symptoms such as fatigue, nausea, and anorexia (b) fluid overload unresponsive to diuretics (c) hyperkalemia >5.5–6.5 meq/L, without electrocardiogram (ECG) changes (d) mild uremic encephalopathy such as confusion and increased sleepiness. Patients with acute kidney injury, acute on chronic kidney disease or those with severe pulmonary edema requiring assisted ventilation, hyperkalemia with ECG changes or >6.6 meq/L, severe metabolic acidosis with pH <7.0, severe uremic encephalopathy were excluded. Patients with a willing kidney donor at the time of presentation were also excluded.
Admission data were obtained from records department. The study was approved by the hospital Ethics Committee, and informed consent was taken from all patients or relatives.
Peritoneal dialysis protocol
Peritoneal access was established by percutaneous placement of a temporary PD catheter by nephrologists using a trocar introduced paramedially on the left or right periumbilical abdominal wall. Exchanges of 2 L PD fluid ‑ 1.7% (Nirlife) (Na, 135 mEq/L; Ca, 3.5 mEq/L; K, 0 mEq/L; Mg, 1.5 mEq/L; lactate, 40 mEq/L; 1.7% glucose) were performed by appointed PD nurses. Patients with fluid overload were managed with hypertonic fluid (2.5% or 4.25% glucose). Two‑liter exchanges were used with 35–50 min dwell time (a total of 36–44 L per day and 18–22 exchanges per day) initially for a period of 24 h. Patients who had improvement in their initial uremic signs and symptoms were continued on PD for a total of 48 h and were discharged immediately thereafter. Those with persistent or worsening uremic signs and symptoms or PD‑related complication, namely pain unresponsive to conservative treatment with analgesics, severe hemorrhage requiring blood transfusion, peritonitis, bowel, or bladder injury and poor outflow were switched to HD.
Those who successfully completed the first session of PD were grouped into three categories based on the time gap till the second session of PD. Group 1 included patients who underwent a second PD within 7 days, Group 2 who underwent PD within 8–14 days and Group 3 required PD within 15–21 days. Subsequent session of PD was performed using the technique described above.
Baseline demographic and clinical data included age, sex, mean arterial pressure (MAP), primary kidney disease, body mass index (BMI), and urine output. Biochemical data collected included hemoglobin (HB), serum albumin, serum creatinine, blood urea, serum calcium, phosphate, cholesterol, triglycerides, and intact parathyroid hormone (iPTH). eGFR was measured using the modification of diet in renal disease formula.
The patients were followed till death, renal transplantation, and switch to HD for any reason or for 12 months after initiation of PD.
Statistical analysis
The results are expressed in frequencies and percentages (categorical variables), mean (standard deviation) and medians with interquartile range (skewed distribution). The mean were compared using one‑way ANOVA. Variables with P < 0.05 were considered significant. The end point for calculating death‑censored technique survival was a switch to HD for any reason. The censored events were renal transplantation, death, still on PD after 12 months and lost to follow‑up. Survival curves were generated using Kaplan–Meier method. All statistics were done using SPSS software application (version 20: SPSS, Chicago, IL, USA).
Results
Results
Baseline demographic and clinical characteristics
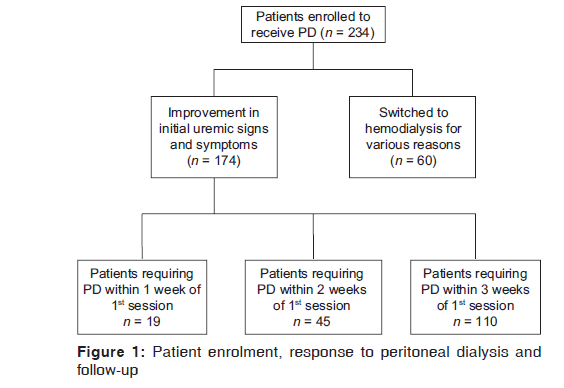
A total of 234 patients have included in the study of which 174 patients had a good response to the initial session of PD. Sixty patients were switched to HD during the first session of PD [Figure 1]. The demographic and clinical characteristics of the two groups are shown in Table 1. Patients who tolerated PD well were older with a higher BMI and serum albumin.
| Variable | Good response Unresponsive | P | |
|---|---|---|---|
| to PD | to PD | ||
| Patients | 174 | 60 | |
| Age (years) | 43.6 (7.3) | 39.3(8.9) | <0.001 |
| BMI (kg/m2) | 23.3 (2.7) | 21.5(2.2) | <0.001 |
| Sex | |||
| Male | 116 | 31 | |
| Female | 58 | 14 | |
| MAP (mm Hg) | 118.3 (14.6) | 129.2(12.8) | <0.001 |
| Primary kidney disease | |||
| Diabetes mellitus | 53 | 13 | |
| Nephrolithiasis | 30 | 10 | |
| Hypertension | 29 | 11 | |
| Chronic glomerulonephritis | 28 | 10 | |
| Chronic interstitial nephritis | 20 | 8 | |
| ADPKD | 9 | 5 | |
| Others | 5 | 3 | |
| HB (g/dl) | 8.2 (0.96) | 8.1 (1.22) | 0.52 |
| Albumin (g/L) | 3.5 (0.29) | 3.37 (0.54) | 0.02 |
| Calcium (mg/dl) | 7.64 (0.54) | 7.59(0.7) | 0.57 |
| Phosphate (mg/dl) | 5.7 (1.9) | 5.9 (2.1) | 0.49 |
| Potassium (mg/dl) | 4.09 (0.87) | 4.9 (1.1) | <0.001 |
| iPTH (pg/ml) | |||
| Median | 480 | 438 | |
| Range | 37-1066 | 168-823 | |
| Cholesterol (mg/dl) | 193.8 (52.6) | 186.8(67.1) | 0.40 |
| TG (mg/dl) | 162 (105.4) | 156.6 (110.3) | 0.73 |
| Creatinine (mg/dl) | 9.5 (4.1) | 9.3 (3.7) | 0.63 |
| Urea (mg/dl) | 134.5 (37.1) | 148.4(52.3) | 0.02 |
| eGFR (ml/min/1.73 m2) | 9.7 (3.8) | 10.8(4.6) | 0.06 |
| Urine output (ml) | |||
| Median | 750 | 350 | |
| Range | 100-1400 | 150-900 | |
BMI: Body mass index, eGFR: Estimated glomerular filtration rate, TG: Triglycerides, iPTH: Intact parathyroid hormone, MAP: Mean arterial pressure, ADPKD: Autosomal dominant polycystic kidney disease, PD: Peritoneal dialysis, HB: Hemoglobin
Table 1: Characteristics of patients initially subjected to PD
Following the initial session of PD, patients were divided into three groups. Nineteen patients required a second session of PD within 7 days of the first session (Group 1, mean age 45.4 [3.2] years), 45 patients within 7–14 days of the first session (Group 2, mean age 46.2 [6.5] years) and 110 patients within 15–21 days (Group 3, mean age 42.2 [8.3] years) [Table 2]. The most common cause of ESRD was diabetes mellitus in all the three groups; nephrolithiasis was the second most common cause, followed by hypertension, chronic glomerulonephritis and chronic interstitial nephritis. The MAP was significantly higher in the group with a longer gap between two sessions of PD (P < 0.001). The BMI was significantly higher in Group 3 compared to the other two groups (P < 0.01).
| Variable | Group 1(within 1 week) | Group 2(within 2 weeks) | Group 3(within 3 weeks) | P |
|---|---|---|---|---|
| Patients | 19 | 45 | 110 | |
| Age (years) | 45.4 (3.2) | 46.2(6.5) | 42.2(8.3) | <0.01 |
| BMI (kg/m2) | 21.2(2.01) | 23.5(3.1) | 23.6(2.6) | <0.01 |
| Sex | ||||
| Male | 12 | 31 | 73 | |
| Female | 7 | 14 | 37 | |
| MAP (mm Hg) | 115.3 (14.2) | 111.7(13.7) | 121.5(15.1) | <0.01 |
| Primary kidney disease | ||||
| Diabetes mellitus | 7 | 15 | 31 | |
| Nephrolithiasis | 4 | 7 | 19 | |
| Hypertension | 4 | 8 | 17 | |
| Chronic glomerulonephritis | 3 | 7 | 18 | |
| Chronic interstitial nephritis | 6 | 14 | ||
| ADPKD | 1 | 2 | 6 | |
| Others | 5 | |||
| HB (g/dl) | 7.97(1.03) | 7.84(1.1) | 8.4 (0.9) | <0.01 |
| Albumin (g/L) | 3.21(0.35) | 3.4 (0.29) | 3.6 (0.24) | <0.001 |
| Calcium (mg/dl) | 7.11(0.61) | 7.5 (0.48) | 7.79 (0.56) | <0.001 |
| Phosphate (mg/dl) | 4.77(1.46) | 5.74 (1.57) | 5.82(2.1) | 0.09 |
| iPTH (pg/ml) | ||||
| Median | 196 | 480 | 570 | |
| Range | 37-1066 | 267-784 | 47-920 | |
| Cholesterol (mg/dl) | 191.8 (53.6) | 194.9(52.8) | 193.7(52.2) | 0.93 |
| TG (mg/dl) | 146.9(100.2) | 161.06(107.9) | 165.2 (105.3) | 0.78 |
| Creatinine (mg/dl) | 8.4(3.4) | 9.1 (3.4) | 9.8 (4.5) | 0.31 |
| Urea (mg/dl) | 121.3 (35.8) | 131.8(38.3) | 137.9(36.9) | 0.17 |
| eGFR (ml/min/1.73 m2) | 7.87 (4.1) | 9.4 (4.3) | 10.2(3.5) | 0.04 |
| Urine output (ml) | ||||
| Median | 450 | 650 | 750 | |
| Range | 100-800 | 200-1000 | 200-1400 |
BMI: Body mass index, eGFR: Estimated glomerular filtration rate, TG: Triglycerides, iPTH: Intact parathyroid hormone, MAP: Mean arterial pressure, ADPKD: Autosomal dominant polycystic kidney disease, PD: Peritoneal dialysis, HB: Hemoglobin
Table 2: Demographic and clinical characteristics of patients based upon the requirement of second session of PD
The laboratory data showed significant difference in HB (P < 0.01), serum albumin (P < 0.001), and serum calcium (P < 0.001) with values being significantly higher in Group 3 compared to Group 1 and Group 2. The median value of iPTH was also higher in Group 3. Total urine output in 24 h at start of dialysis was higher in Group 3. The eGFR (P = 0.04) was significantly higher in Group 3.
Outcomes
By the end of the study, 91/174 (52%) patients were still receiving repeated puncture PD with 5/19 (26.3%) in Group 1, 21/45 (46.7%) in Group 2 and 65/110 (59.1%) patients in Group 3 which was statistically significant (P = 0.05) [Table 3]. The median symptom‑free period was 23 days in Group 3 compared to 11 days in Group 1. A total of 42 patients had to be switched to HD for various reasons, with the complications relating to PD (47.6%, 20/42), as the most common reason followed by persistence of uremic symptoms (42.9%, 18/42) even after completing a session of PD. Mechanical failure due to either inadequate drainage or difficulty in placing PD catheter resulted in 4 patients (9.5%, 4/42) being shifted to HD. Fourteen patients underwent renal transplantation during the follow‑up, while 14 patients died, with infection and sepsis as the most common cause. 12 patients were lost to follow‑up.
| Variable | Overall | Group 1 (n=19) | Group 2 (n=45) | Group 3 (n=110) | P |
|---|---|---|---|---|---|
| Still on PD | 91 | 5 | 21 | 65 | 0.04 |
| Total sessions of PD | 1932 | 174 | 469 | 1289 | |
| Median dialysis free days (range) | 11 (5-28) | 16 (10-31) | 23 (10-41) | ||
| Switched to HD | 42 | 5 | 16 | 21 | |
| Reason | |||||
| Complications | 20 | 1 | 4 | 6 | |
| Persistence of symptoms | 18 | 3 | 11 | 13 | |
| Mechanical failure | 4 | 1 | 1 | 2 | |
| Death | 14 | 4 | 2 | 8 | |
| Transplantation | 14 | 3 | 4 | 7 | |
| Lost to follow-up | 12 | 2 | 2 | 8 | |
PD: Peritoneal dialysis, HD: Hemodialysis
Table 3: Outcomes of the patients at study end
Peritoneal dialysis related complications
A total of 2261 sessions of PD were recorded. The majority of the patients (63.5%, 1431/2261) experienced pain during inflow which was managed with keeping the small volume of initial few exchanges and slowing the inflow rate. Only a few patients required analgesic for the pain. The other common complication was pericatheter leak (11.3%, 256/2261) which again managed with low volume exchange. Initial hemorrhagic drainage was noticed in 124 (5.5%, 124/2261) sessions. In most of these, the drainage became clear after 5–6 rapid exchanges and no blood transfusion was required. In 6 patients, hemorrhage was severe resulting in discontinuation of PD and patients were switched to HD. 49 episode of peritonitis occurred with the most common organism being Escherichia coli. Most of these occurred in patients who had multiple manipulation of PD catheter. Most of the cases were responsive to intravenous antibiotics. Eleven patients with severe peritonitis unresponsive to antibiotics had to be switched over to HD [Table 4]. Bowel perforation occurred in 2 patients.
| Total number of session | n=2261 (%) |
|---|---|
| Pericatheter leak | 256(11.3) |
| Pain | 1431 (63.5) |
| Bleeding | 124 (5.5) |
| Cessation of PD due to excessive haemorrhage | 6 |
| Bowel perforation | 2 (0.09) |
| Peritonitis | 49(2.1) |
| Mechanical problems | 62(2.7) |
IPD: Intermittent peritoneal dialysis
Table 4: Complications during IPD
Death‑censored technique survival
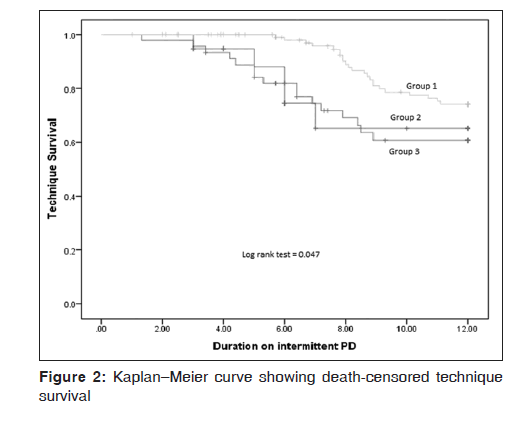
The overall 1 year death‑censored technique survival was 68.4% (91/133). The rates were 50% (5/10), 56.8% (21/37), and 75.6% (65/86) in Group 1, Group 2, and Group 3, respectively. The mean survival time on PD before switching to HD was 9.8 months in group 1 (95% confidence interval [CI]: 8.1–11.4 months), 9.6 months (95% CI 8.6–10.6 months) in Group 2, and 11.1 months (95% CI: 10.7–11.4 months) in Group 3. On Kaplan–Meier analysis, a significant difference was observed in death‑censored technique survival between the three groups (log rank [Mantel‑Cox] = 0.05) [Figure 2].
Discussion
The practice of RRT in India is guided mainly by economic considerations. Unlike the developed countries, there is no registry of patients with ESRD in India. Hence, outcomes of these patients are largely unknown. Most of dialysis center are privately managed and are located in large cities. Thus, for a patient requiring dialysis support in a rural area, this requires a lot of investment in terms of time and money. The adherence to the dialysis program is often poor. Rao et al. noted that almost 60% of their patients left the RRT program largely due to financial constraints.[4] In their series, only 3.6% of the patients remained on HD after 1 year and 4.5% on CAPD. The mortality in their cohort was 9.5%.[4] This was seconded by Abraham et al. who noticed a dropout rate of 51% within 2 years of starting CAPD.[5] Often patients fail to reach the dialysis center at the time of crisis. To bridge some of these gaps and ensure adequate compliance, an alternative, affordable form of RRT is needed. This is where intermittent PD (IPD) as a mode of RRT can be useful. Although IPD is biochemically inferior to HD, the minimal infrastructure and operation cost, need for highly trained staff and sophisticated equipment, makes it an attractive mode of RRT, especially in areas with low‑resources.
PD has often been described as “the poor stepchild in therapy for end stage renal disease.” Experiment with peritoneal lavage was first reported by Wegner in 1877.[6] However it was in 1923 when Georg Ganter published the first trials of PD for the management of uremia, which prompted researchers around the world to focus on PD as a mode of treating uremia, which until then was a “death sentence” for the patient. In 1946, Frank et al. reported the first successful use of “peritoneal irrigation” in the treatment of acute renal failure.[7] Various review during 1950’ demonstrated that patients treated with PD showed clinical and biochemical improvement. However, the high incidence of peritoneal infection after repeated puncture PD and other complications associated with it meant that the use of PD was limited to patients who had a reversible cause of their renal failure.[7]
The use of IPD was practiced widely in the 1970’s mostly in patients with acute kidney injury; however, its use in ESRD was debatable.[7] The use of repeated puncture IPD has declined in the recent years in favor of intermittent HD and continuous RRT. It lacks the efficiency of HD, the clearance per exchange decreases with shorter dwell, and ultrafiltration rate cannot be controlled as fluid removal is limited and the possibility of PD worsening mechanical ventilation.[8‑10]
Since this practice of IPD is often considered “old,” studies comparing with other standard forms RRT are lacking. IPD as a form of RRT was studied in the 1960’s and 70’s.[11‑13] In a formal program, 30 nonoliguric patients were offered repeated PD whenever there was biochemical or clinical deterioration. Survival for 4 months was unusual.[12] All these studies are small and for a short duration. In our study, the gap between the first two sessions of PD proved to be a reliable indicator of the further course for the patient on periodic PD. A significant number of patients could be maintained on IPD after 1 year. Those who had an earlier deterioration of their clinical and biochemical status requiring early institution of PD had a lower technique survival. These patients had a lower BMI, albumin level, and HB levels, signifying malnutrition or a persistent inflammatory state. They also had a lower creatinine clearance and a lower urine output. These factors may help to decide the patient profile which can be maintained of repeated puncture PD. Similarly, a longer gap between the first two sessions of PD meant that the patient had a better subsequent course. These patients were maintaining a dialysis‑free period of 23 days. Frank peritonitis rate was very low compared to earlier studies. One reason could be the prophylactic use of broad spectrum antibiotics for the session of PD, however, whether this can be considered a significant factor is debatable. No death occurred as a direct consequence of PD. The mortality rate in our study was 8% which is comparable to those seen in other forms of RRT. A mortality rate of 20% within 2 years of initiating HD was reported by Singh and Bhandari.[14]
These data are significant as it provides a cost effective way of managing uremia in patients who cannot be maintained with other conservative measures and is particularly useful for low socioeconomic patients without a potential donor, who otherwise would be destined to die a miserable life. As discussed earlier, this form of PD is provided free of cost to patients in government sector hospital across Rajasthan, thus its utility in this part of the world has a greater significance. Even otherwise, a single session of PD would cost approximately Rs. 2000, and would benefit particularly those patients who require a session every 2 or 3 weeks.
There are certain limitations of this study. First, since, it’s a single center study; it cannot be used to represent the overall situation across India or developing world. A multi‑center study is required to validate the issue. Secondly, a control group was lacking for comparison with other standard form of therapy such as HD or CAPD. Thirdly, PD adequacy could not be measured due to lack of required data.
Conclusion
Our study showed that it is possible to maintain patients with terminal uremia on repeated puncture PD, without significant complications and provides a safe and cost effective way of palliation in these patients. In spite of limitations, PD can be successfully accomplished in remote areas and in low resource areas.
Acknowledgement
We would like to thank the nursing staff of Sawai Man Singh Hospital for their contribution to the peritoneal dialysis program.
Financial support and sponsorship
Nil.
Conflict of Interest
There are no conflicts of interest.
REFERENCES
- Modi G, Jha V. Incidence of ESRD in India. Kidney Int 2011;79:573.
- Modi GK, Jha V. The incidence of end‑stage renal disease in India: A population‑based study. Kidney Int 2006;70:2131‑3.
- Jha V. Current status of end‑stage renal disease care in India and Pakistan. Kidney Int Suppl 2013;3:157‑60.
- Rao M, Juneja R, Shirly RB, Jacob CK. Haemodialysis for end‑stage renal disease in Southern India – A perspective from a tertiary referral care centre. Nephrol Dial Transplant 1998;13:2494‑500.
- Abraham G, Kumar V, Nayak KS, Ravichandran R, Srinivasan G, Krishnamurthy M, et al. Predictors of long‑term survival on peritoneal dialysis in South India: A multicenter study. Perit Dial Int 2010;30:29‑34.
- Oreopoulos DG, Thodis E. The history of peritoneal dialysis: Early years at Toronto western hospital. Dial Transplant 2010;39:338‑43.
- Frank HA, Seligman AM, Fine J. Further experiences with peritoneal irrigation for acute renal failure: Including a description of modifications in method. Ann Surg 1948;128:561‑608.
- Ponce D, Berbel MN, Regina de Goes C, Almeida CT, Balbi AL. High‑volume peritoneal dialysis in acute kidney injury: Indications and limitations. Clin J Am Soc Nephrol 2012;7:887‑94.
- Ponce D, Buffarah MB, Goes C, Balbi A. Peritoneal dialysis in acute kidney injury: Trends in the outcome across time periods. PLoS One 2015;10:e0126436.
- Ponce D, Berbel MN, Abrão JM, Goes CR, Balbi AL. A randomized clinical trial of high volume peritoneal dialysis versus extended daily hemodialysis for acute kidney injury patients. Int Urol Nephrol 2013;45:869‑78.
- Asghar RB, Bandyopadhay S, Woywodt A. Intermittent peritoneal dialysis: Just enough for some or inadequate altogether? Perit Dial Int 2012;32:134‑6.
- Edelbaum DN, Sokol A, Gaynor S, Rubini ME. Peritoneal dialysis in chronic renal failure. Calif Med 1968;108:85‑9.
- Fourtounas C, Hardalias A, Dousdampanis P, Savidaki E, Vlachojannis JG. Intermittent peritoneal dialysis (IPD): An old but still effective modality for severely disabled ESRD patients. Nephrol Dial Transplant 2009;24:3215‑8.
- Singh P, Bhandari M. Renal replacement therapy options from an Indian perspective: Dialysis versus transplantation. Transplant Proc 2004;36:2013‑4.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.