Perioperative Anesthesia Care for an Infant Presented with Fulminant Respiratory Distress Secondary a Congenital Pulmonary Airway Malformation in a Resource-Constrained Area-A Rare Case Report
Received: 08-May-2023, Manuscript No. AMHSR-23-98054; Editor assigned: 11-May-2023, Pre QC No. AMHSR-23-98054 (PQ); Reviewed: 25-May-2023 QC No. AMHSR-23-98054; Revised: 07-Jul-2023, Manuscript No. AMHSR-23-98054 (R); Published: 14-Jul-2023
Citation: Ilala TT, et al. Perioperative Anesthesia Care For an Infant Presented with Fulminant Respiratory Distress Secondary a Congenital Pulmonary Airway Malformation in a Resource-Constrained Area. A Rare Case Report. Ann Med Health Sci Res. 2023;13:867-873
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: Infantile congenital pulmonary airway malformation is considered a rare congenital malformation occurring in infants. The provision of anesthesia for such infants bears a substantially challenging situation, as it may lead to sudden hemodynamic collapse during the induction of anesthesia, and positive pressure ventilation resulting in hyperin lation of the cystic lesion. Effective airway management requires thorough knowledge and experience in one-lung ventilation and recognition of related complications during pneumonectomy or lobectomy. Case description: A 4 months old male infant presented with worsening fast breathing and grunting of two weeks duration. He was referred from another primary hospital to our institution with the diagnosis of type-I cystic congenital airway malformation and severe acute malnutrition (severe wasting). The infant was in fulminant respiratory distress on oxygen supplementation (nasal cannula, at 5 L/ min) on admission. Chest CT scans con irmed the presence of a large multilocular air-illed cystic lesion in the right lower lung likely congenital cystic adenomatoid malformation type-I. Following a detailed discussion with the patient's family about the risk and bene its of surgical anesthesia, written informed consent was obtained general anesthesia with endobronchial intubation (one lung ventilation) was planned for the right pneumonectomy. The infant was induced with propofol, and direct rigid laryngoscopy was performed to place the endotracheal tube into the left main bronchus, and it was difficult to place the endotracheal tube into the left main bronchus correctly by blind technique on the irst attempt. The infant was desaturated, and we managed it with gently assisted ventilation. Then after managing hypoxia, we use the small size lexible cystoscope to place the endotracheal tube into the left main bronchus. Finally, the correct placement of the left main bronchial tube was achieved with the lexible cystoscopy passed through the 3.5 mm ID cuffed standard endotracheal tube. Conclusion: Surgery may be indicated in higher stages of the lesion, and this may bear a greater challenge on anesthesia management, particularly airway management is critical during one lung ventilation. Lung separation may possess greater challenges and the use of sophisticated devices aiding in proper lung separation, and used for lung separation might solve or minimize the challenges related to one lung ventilation.
Keywords
Anesthesia; Congenital pulmonary airway malformation; Infants; Lobectomy; Surgery
Abbreviations
CCAM: Congenital Cystic Adenomatoid Malformation; CPAM: Congenital Pulmonary Airway Malformation; OLV: One Lung Ventilation; ICU: Intensive Care Unit
Introduction
Congenital Pulmonary Airway Malformation (CPAM) is a rare developmental abnormality of the lower respiratory tract. This rare entity might result from an embryologic insult in early gestation causing mal development of the terminal bronchiolar structures, which is assumed to develop during the pseudo glandular and saccular period of pulmonary airway development [1]. It is rarely self-limited and over 50% of these infants needs surgical intervention. It is suggested to have an estimated incidence of 1:7, 200 to 1:35,000 and it represents up to 25% of congenital lung abnormalities [2].
It affects the newborn having a similar occurrence in preterm and term infants. This congenital pulmonary airway malformation usually occurred unilaterally and limited to one lobe [3]. This congenital entity may comprise of cystic components and adenomatous elements, which arise from the respiratory tract including trachea, bronchus, bronchiolar, or alveolar tissue. It is characterized by overgrowth of terminal bronchioles with reduced alveoli which do not participate in gas exchange. Congenital pulmonary airway malformation is classified on the basis of the cyst size (type I: Cysts 2 cm-10 cm; type II: Cysts 0.5 cm-2 cm; type III: Micro-cystic) [4]. The lesion is connected to the airway, with a missing normal intrapulmonary bronchial system. Thus, large lesions may cause mediastinal shift, cardiac and caval compression with resultant polyhydramnios, and hydrops fetalis [5]. The mortality is high with 12.5% death rate in newborns, particularly with higher stages of the congenital anomaly and co-existing of other lesions.
The congenital malformation might be diagnosed early during pregnancy with the aid of prenatal sonography. It may decrease or increase in size and manifest as respiratory distress at birth. It may remain asymptomatic if it is small size, and may arise late in life, or occur incidentally [6].
The infants with congenital pulmonary airway malformations might usually present with respiratory insufficiency resulting distress, intercostal and sub-costal retraction, common cold, grunting, and recurrent cough. The cystic expansion and mechanical compression of the pulmonary structures may lead to pulmonary hypoplasia, mediastinal shift increasing the risk of spontaneous pneumothorax [7].
The risk of other associated congenital anomalies including the cardiac, anorectal, renal, intestinal, cardiac, and bone or radial dysplasia is high, which might affect up to 25% of the patients with congenital pulmonary airway malformation complicating the medical, surgical, and anesthesia management especially with the increasing the grade of anomaly.
Except for cardiac anomalies, the other associated malformations worsen the prognosis. Congenital pulmonary airway malformation usually requires surgery in the form of cystectomy or lobectomy or thoracotomy and excision of lesions [8].
The induction of general anesthesia may lead to sudden hemodynamic instability, and Positive Pressure Ventilation (PPV) may cause respiratory embarrassment. Lung isolation is crucial to allow a good surgical access and protect the normal lung from contralateral contamination, which is technically challenging in the absence of proper equipment’s (double lumen tubes, pediatrics bronchial blockers). Particularly, one lung ventilation in infants during thoracotomy for resection of the congenital pulmonary airway malformation is a distressing condition.
We presented a case of fulminant respiratory distress from congenital pulmonary airway malformation and severe malnutrition (severe wasting) who underwent thoracotomy and right lower and portion of middle lobe resection in a resource-constrained area.
Case Presentation
A 4 months old male infant, (45 cm, 5.7 kg), presented with worsening fast breathing and grunting of two weeks duration. He was referred from another primary hospital to our institution with the diagnosis of type-I cystic congenital airway malformation and severe acute malnutrition (severe wasting). He was on the supplemental oxygen (nasal cannula) for three days before transferred to our institution.
On admission, the infant was in fulminant respiratory distress on oxygen (nasal cannula, 5 L/min), from a congenital pulmonary airway malformation referred from other primary hospital to our institution surgical outpatient department. He was delivered at primary hospital through spontaneous vaginal delivery, at 37 weeks of gestation. At birth with one minute Apgar score of 9/10, and 5 minutes Apgar score of 10/10. There was no newborn and neonatal complication. Two months after delivery, he had developed fast breathing, and intermittent difficulty of breathing at which his family visited the nearby hospital, and prescribed him unknown medication. He had history of failure to weight gain despite breast feeding and additional bottle feeding with gradual significant weight loss. His mother has history of full antenatal care services and was uneventful.
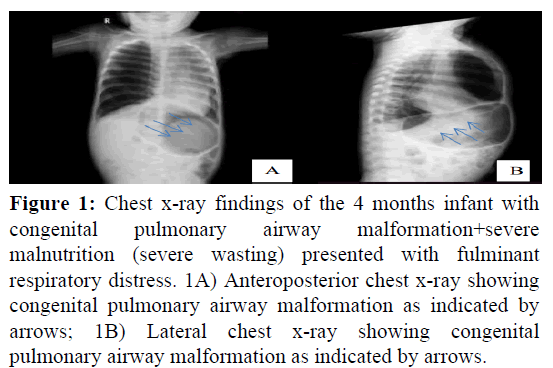
At admission to our institution, he is in the fulminant respiratory distress, and on oxygen supplementation (nasal cannula, at 5 L/min). The infant was in acute sick looking (labored breathing) in respiratory distress on 100% oxygen via nasal cannula. The vital signs of the infant were blood pressure 79/42 mmHg, pulse rate 133 beats per minutes, respiratory rate 50 breaths per minutes, temperature 37.1℃ and peripheral oxygen saturation of (87% on room air), and (95% on nasal cannula with 100% O2). The anthropometric parameters were: Weight=5.7 k; Length (L)=65 cm; Height (H)=45 cm. Weight for age and length for age is (between 0 and 2 standard deviation) within normal limits. Weight for length is less than 3 standard deviation (severe wasting). The infant was in acute respiratory distress (peripheral cyanosis, nasal flaring, tachypnea, intercostal and subcostal retraction, chest in drawing, bulged chest (pectus carinatum), hyper resonant percussion note, and significantly decreased air entry and fine crepitation over the right lower chest, there is scattered wheeze over the lower antero-posterior field of the right lung, and on the supplemental intranasal oxygen. Bulged precordium (pectus carinatum), first heart sound and second heart sound is well heard. No murmur and gallop. The infant was irritable. Laboratory test results depicted that total white blood cells count 7.7 × 103/L (3.6-10.2) × 103/L, red blood cells count; 5.82 × 103/L (4.06-5.36) × 103/L, hemoglobin-13.7 g/dl, hematocrit-42.3%, platelet count of 417 × 103/L (152-348 × 103/L), and erythrocyte sedimentation rate of 5 mm/hour (up to 10 mm/hour). Normal liver and renal function test. Serum electrolyte within normal range. Random blood sugar of 113 mg/dl (70-140) mg/dl. Blood group and Rh=O positive. The radiographic investigation shows the normal echocardiography and electrocardiography report. The chest x-ray was suggestive of congenital emphysema (Figures 1A and 1B).
Figure 1: Chest x-ray findings of the 4 months infant with congenital pulmonary airway malformation+severe malnutrition (severe wasting) presented with fulminant respiratory distress. 1A) Anteroposterior chest x-ray showing congenital pulmonary airway malformation as indicated by arrows; 1B) Lateral chest x-ray showing congenital pulmonary airway malformation as indicated by arrows.
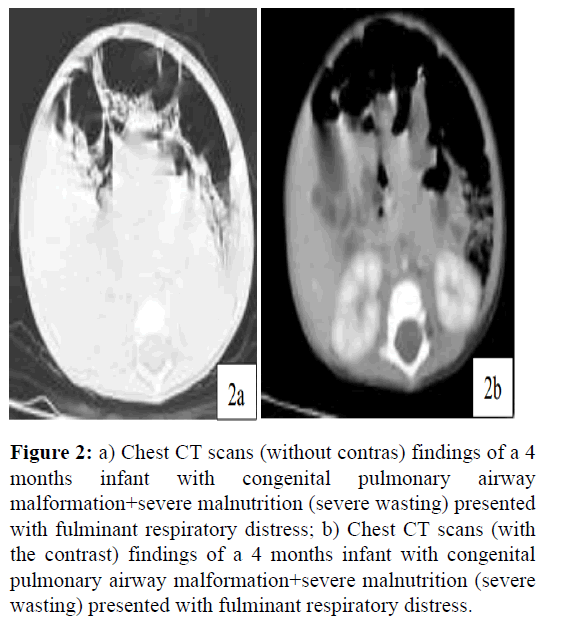
Chest CT scans suggest a large right side chest hemi thorax air filled cystic lesion with thin wall occupying almost the entire right lung and has a mass effect with mediastinal shift to the left side. There is also partial collapse of the remaining lung parenchyma, which concluded as a large multilocular air filled cystic lesion in the right lower lung likely Congenital Cystic Adenomatoid Malformation (CCAM) type-I (Figures 2a and 2b).
Figure 2: a) Chest CT scans (without contras) findings of a 4 months infant with congenital pulmonary airway malformation+severe malnutrition (severe wasting) presented with fulminant respiratory distress; b) Chest CT scans (with the contrast) findings of a 4 months infant with congenital pulmonary airway malformation+severe malnutrition (severe wasting) presented with fulminant respiratory distress.
After all necessary workup was made his final assessment was: ASA-III+infant airway+congenital pulmonary airway malformation+severe malnutrition (severe wasting) and we decided to be fit for surgery and anesthesia. The night before day of operation, he was reassessed by the senior anesthetist, the risk of surgery and anesthesia was discussed clearly with the patient’s parents. Then after written informed consent was obtained, we planned to provide general anesthesia with endobronchial intubation (one lung ventilation) for the procedure of right pneumonectomy.
On the morning of the day of the surgery, informed consent was confirmed, and the patient was taken to the operation with the intranasal oxygen in place as Figure 3.
The in situ 22G IV cannula was checked and isotonic saline was given at a rate of 30 drop/minutes. Intra-intravenous atropine (15 mcg/kg) and dexamethasone 0.15 mg/kg was injected. Paracetamol 20 mg/kg was administered per rectal (suppository). The American society of anesthesiologist standard monitors were applied: (Capnography, indirect blood pressure measurement cuff, pulsi-oxymetry, electrocardiography (on the back) and axillary temperature probe), and precordial stethoscope.
The infant was positioned in semi lateral (right) position to minimize the respiratory compromise, exacerbated in supine. Following sufficient pre-oxygenation with high flow fraction of oxygen at 10 L/minutes with circle breathing system, Induction of general anesthesia was conducted via intravenous route, with the slow injection of propofol (3 mg/ kg), then loss of consciousness was confirmed and short acting muscle relaxant administered. Plain lidocaine was injected at 1.5 mg/kg to obtund airway response to airway instrumentation. Positive pressure ventilation was avoided to mitigate the risk of spontaneous or exacerbation of pneumothorax, and possible expansion of cystic contents. Attempt at endotracheal tube placement was made deemed that adequate depth of anesthesia was achieved, and addition of short acting muscle relaxant (IV succinylcholine, 1.5 mg/kg) to smooth the course of induction and intubation process.
On the first attempt at intubation, direct rigid laryngoscopy was performed and styleted 3.5 mm ID endotracheal tube was passed the cord under direct vision and slightly rotated in clockwise direction, advanced to the left main bronchus with correct placement confirmed with auscultation. Thus, it was challenging that correct placement of standard endotracheal tube in the left main bronchus was not an eventful, and the infant desaturate which renders us to provide positive pressure ventilation. It was not possible to load the endotracheal tube of small size over the rigid bronchoscope, and there is no flexible fiber optic bronchoscope which is ideal for aiding for intubation and confirmation of endobronchial tube placement during lung separation procedures we attempted blind endobronchial intubation. The rapid desaturation following the first attempt (after 45 to 60 seconds) responded to gentle assisted ventilation. Then after managing desaturation (we achieved oxygen saturation 100%), 5 mg of propofol and 0.25 mg/kg of succinylcholine was added to ensuring adequate anesthesia and muscle relaxation, and the second attempt at endobronchial intubation was performed. The experienced pediatric surgeon addressed important idea on the use of small size flexible cystoscope to overcome this clinical problem, which we have no idea before. Then, we use the flexible cystoscope, and following the repeated attempt at intubation, correct placement of the left main bronchial tube was achieved with the use of 17 Fr (size) flexible cystoscopy, passed through the 3.5 mm ID cuffed standard endotracheal tube. Then after we confirmed the correct placement of the endobronchial tube, we shifted the maintenance of anesthesia to halogenated anesthetics. We withhold the use of gaseous induction to minimize the subsequent risk of cystic expansion or rupture as a result positive pressure ventilation, and iatrogenic pneumothorax as this decrease the perils of respiratory compromise following induction of anesthesia.
Intermediate acting muscle relaxant was administered, and artificial ventilation was initiated with the lung protective ventilation strategy with volume controlled mode. Following the lung separation, tidal volume (6 ml/kg), and (2-3) mmHg PEEP was provided with the increasing of respiratory rate (15-20)% of two lung ventilation. After we stabilize the patient ventilation and hemodynamic status, the infant was placed in the left lateral position.
Right thoracotomy was done by right posteromedial chest incision was made at the 5th intercostal space and pleural cavity was sutured in layer, by sparing latissimus and serratus anterior muscles. The large air filled cystic lesion on the right lower lobe was punctured and decompressed. Right lower lobectomy was done after lower lobe is mobilized proximally and the structure is identified. The segmental arteries, veins, and bronchi ligated separately. A 14Fr chest tube was placed and chest wall closed in layer. During the intraoperative course there was repeated intermittent events of hypoxia, which was managed by withdrawal of the standard endotracheal tube inserted deep to the bronchus to the tracheal and ventilation of both lungs as it is not possible to apply continuous positive airway pressure to the nondependent lung with single lumen standard tracheal tube. After managing hypoxia with two lung ventilation, The ETT was passed replaced back into the left main bronchus by using flexible cystoscope. Apart from hypoxia, there was no significant hemodynamic event during intraoperative period. The duration of operation was 120 minutes and the total time of anesthesia was 150 minutes. A total volume of 280 ml of 0.9% saline was given. Intraoperative urine output was 0.7 ml/kg/hr.
Post-operatively, the infant was transferred to the intensive care unit with endotracheal tube in place the endotracheal tube was withdrawn and secured in the trachea (maintained in correct position) for post-operative respiratory support, continuous close monitoring and intensive intervention. Immediately, following admission to the intensive care unit, standard monitors were attached (pulsi-oximetry, blood pressure monitors, thermometer), and serial urine output measurement were employed. Pressure controlled, with synchronized intermittent ventilation was initiated, till the infant respiratory effort was regained (after three hours of intensive care unit admission). Postoperative analgesia was achieved with multimodal analgesia. A broad spectrum antibiotic was initiated in the intensive care unit to combat wound infection. The infant was weaned from artificial ventilation and extubated after 12 hours of intensive care stay. On the second day the infant was transferred to surgical pediatric ward with improvement after 48 hours of intensive care stay.
The infant was discharged to home after 8 days of hospital admission with normal serial chest X-ray report and resolved chest condition, and normal breathing and respiratory pattern. On home discharge the infant parents have given advice toward the indicators of any medical problem including respiratory related complications, and to have medical visit early in case.
Results and Discussion
This is rare congenital lung anomalies that result in cystic or adenomatous pulmonary field. The affected infants may commonly present with respiratory compromise leading to respiratory distress, chest retraction, grunting, common cold and recurrent cough which is typical presentation of the current case. The cystic expansion and mechanical compression of the pulmonary structures may lead to pulmonary hypoplasia, mediastinal shift increasing the risk of spontaneous pneumothorax.
It may be managed conservatively or through surgical interventions depending on the clinical manifestation and stages of the disease. Early diagnosis, and intervention is crucial to provide a better medical, and surgical management as in the present case to minimize mortality and improve the outcome [9]. Some setups follow conservative approach for asymptomatic patients, and consider surgery only after symptoms started to manifest (which is called expectant therapy) [10,11]. Treatment options for prenatal care in those fetuses with congenital pulmonary airway malformation, who diagnosed prenatally were administering steroids to the mother, doing minimally invasive procedures, or performing open surgery on the fetus [12].
However, elective prophylactic excision of asymptomatic congenital pulmonary airway malformation lesions in contrast to expectant therapy may benefit the patient, because the lung resection in those asymptomatic patients is found to be quite safe, lowers the chance of developing symptoms later in life, and may even prevent the risk of developing cancer. Whereas conservative therapy may reduce the risk of unnecessary surgical trauma. Hence, there may be potential problems with the conservative strategy include the prolonged duration of outpatient supervision and related dropout in outpatient attendance, greater radiographic studies and radiation exposure, as well as the increased risks of emergency surgery for late complications. In the current case, surgery was performed to relieve the respiratory distress unresolved with conservative management, and an infant was fully recovered from suffering of respiratory compromise.
Congenital pulmonary airway malformation commonly requires surgical treatment in the form of lobectomy or cystectomy or thoracotomy and lesion excision. The close serial monitoring of vital signs are mandatory during neonatal, and an infant surgery, particularly during thoracotomy as it impose greatest risk to the neonate or infant. During anesthesia due to positive pressure ventilation there is increased risk of rapid inflation of the cystic content with sudden mediastinal shift leading severe respiratory compromise, and cardiac arrest.
Perioperative anesthesia management of surgery for congenital pulmonary airway malformation in developing countries with resource limited area like our setup is very difficult. For anesthetists, performing thoracic surgery on newborns and babies is challenging and necessitates extensive training in pediatric anesthesia and fiberscope use [13]. In our case, even if we are not familiar with the use of flexible cystoscope before we use it to guide and confirm the correct placement of endotracheal tube into the left main bronchus.
The preferred technique of anesthesia for congenital pulmonary airway malformation of lung resection procedures is general anesthesia as it provides favorable surgical environment with control of airway and hemodynamics, adequate muscle relaxation, amnesia and adequate pain managements as required.
Congenital pulmonary airway malformation cyst and involved lung lobe resection requires separate ventilation of the normal lung from the cystic lung, which termed as One Lung Ventilation (OLV). OLV is essential to protect normal lung from contralateral contamination, to enhance surgical access, to lessen blood loss and to reduce trauma to the residual normal lung tissue [14]. However, OLV in neonates and infants is very challenging especially in resource limited setting or practical environment, where getting proper equipment and experience is limited. This is the challenge we face in the current case management.
Although implementing OLV in an infant is difficult and challenging, it can still be performed using double lumen tube, bronchial blockers, endobronchial intubation, selective main stem intubation or surgical retractions. Hence, the use of appropriate pediatrics OLV techniques (pediatrics bronchial blocker, or double lumen tubes) allows the application of suction, and proper deflation of the lung. However, the current case, we managed OLV by using standard endotracheal tube advanced to the left main stem bronchus, since it was the only option available at the time. The patient was successfully managed with it and the outcome was good.
Similar to the current case, although it is associated with higher incidence of intraoperative hypoxia due to an increased in dead space and obstruction of the upper lobe of the bronchus, the use of single lumen endotracheal tube is desired approach.
During thoracoscopy procedure for congenital pulmonary airway malformation, infants and neonates are more susceptible to hypoxia and hypercarbia issues than older children due to their unique anatomical and physiological characteristics like limited functional residual capacity and the need for OLV. A cohort study by Templeton et al., conclude that during one-lung ventilation, children seem to be at more risk for hypoxemia than adults and the lesser risk of hypoxemia is reported when a bronchial blocker is used as the lung isolation approach compared to endobronchial intubation. Intraoperative hypoxia during OLV was managed by withdrawing the ETT back into the trachea. Similarly, in the current case intraoperative hypoxia was the main challenge, and managed in as similar manner.
Thoracic surgeries such as thoracotomy, lobectomy or cystectomy can be done for patients with congenital pulmonary airway malformation, and might leads to the occurrence of sudden cardiovascular and hemodynamic collapse, which poses the major anesthetic challenge. To prevent bronchial stump dehiscence caused by iatrogenic ventilator use, early extubation should be taken into consideration. In the present case, the extubation was done after 12 hours of Intensive Care Unit (ICU) stay on ventilation support and intensive care.
Conclusion
Effective management of the infant with congenital pulmonary airway malformation needs a multidisciplinary approach, early diagnosis and intervention to minimize mortality and improve outcome. Thus, the CPAM might be managed conservatively or through surgical interventions depending on the clinical manifestation and stages of the disease. Surgical intervention is indicated for higher stages of lesion, and hence it bears a greater challenge on the anesthesia management, particularly critical airway management during one lung ventilation. Lung separation may possess greater challenge us in our case. The use of sophisticated device aiding in proper lung separation like flexible bronchoscope, and devices used for lung separation (bronchial blockers, double lumen tube) might solve or minimize the challenges related to one lung ventilation. However, in the pace of pre and intraoperative respiratory events and airway management challenges we have successfully managed the case.
Ethics Approval and Consent to Participate
We received proper patient consents to participate in this study. The patient family was given their consent for the clinical data to be incorporated in the paper. Confidentiality was maintained throughout, with a due concerns will be made to protect his privacy.
Publication Consent
Written informed consent was provided from the patient family for publication of the case details and images.
Author Contributions
TT contribute in writing the first and final paper draft, manuscript development, conceptualization, methodology, formal analysis, supervision, data validation, curation, and interpretation. GT and FB were playing a great contribution in conceptualization, methodology, formal analysis, and interpretation. MY and AB contribute to writing the draft, formal analysis, fund acquisition, manuscript writing, data validation, supervision and methodology.
Data Availability
Data are available by corresponding author upon reasonable request.
Competing Interest
No.
Funding source
Not applicable.
Acknowledgments
We would like to thank our team members who help and devoted their time in a wise patient management and preparing the paper.
References
- Gupta L, Gupta B, Gupta A, Jain R. Anesthetic considerations in an infant with congenital cystic adenomatoid malformation. Indian J Clin Anaesth. 2020;5:156-158.
- Mahajan S, Dave N, Dias R, Chhabria R. Anesthetic considerations and challenges in infants with congenital pulmonary airway malformation. Pediatr Anesth Crit Care J. 2016;4:108-111. [
- Dias R. Congenital pulmonary airway malformation. Anaes Intens. 2022;63.
- Upadhya RK, Shenoy L, Venkateswaran R. Effect of intravenous dexmedetomidine administered as bolus or as bolus plus infusion on subarachnoid anesthesia with hyperbaric bupivacaine. J Anaesthesiol Clin Pharmacol. 2018;34:46-50.
[Crossref] [Google Scholar] [PubMed]
- Oakes JM. The anesthetic care of an infant with congenital cystic adenomatoid malformation. J Anesth Intensive Care Med. 2017;4:10-12.
- Guruswamy V, Roberts S, Arnold P, Potter F. Anaesthetic management of a neonate with congenital cyst adenoid malformation. Br J Anaesth. 2005;240-242.
[Crossref] [Google Scholar] [PubMed]
- Budzinski J. Occurrence of tension pneumothorax following fog arty embolectomy catheter use for lung isolation in a neonate with congenital pulmonary airway malformation. Int J Anesth Anesthesiol. 2018;5:3-5.
- Liu D, Hu Z. Congenital pulmonary airway malformation. Am J Med Sci. 2023;S0002-S9629.
[Crossref] [Google Scholar] [PubMed]
- Kapralik J, Wayne C, Chan E, Nasr A. Surgical versus conservative management of congenital pulmonary airway malformation in children: A systematic review and meta-analysis. J Pediatr Surg. 2016;51:508-512.
[Crossref] [Google Scholar] [PubMed]
- Makhijani AV, Wong FY. Conservative post-natal management of antenatally diagnosed congenital pulmonary airway malformations. J Paediatr Child Health. 2018;54:267-271.
[Crossref] [Google Scholar] [PubMed]
- David M, Lamas-Pinheiro R, Henriques-Coelho T. Prenatal and postnatal management of congenital pulmonary airway malformation. Neonatol. 2016;110:101-115.
[Crossref] [Google Scholar] [PubMed]
- Caruselli M, Galvagni D, Boubnova J, Michel F. Anesthetic management of pulmonary surgery in newborns and infants. Pediatr Rep. 2020;12:61:63.
[Crossref] [Google Scholar] [PubMed]
- Chiluveru S, Dave N, Dias R, Garasia M. Congenital pulmonary airway malformation with atrial septal defect and pulmonary hypertension for lobectomy anesthetic considerations. Ann Card Anaesth. 2016;19:372-374.
[Crossref] [Google Scholar] [PubMed]
- Templeton TW, Miller SA, Lee LK, Kheterpal S, Mathis MR, Goenaga-Diaz EJ, et al. Hypoxemia in young children undergoing one lung ventilation: A retrospective cohort study. Anesthesiol. 2021;135:842-853.
[Crossref] [Google Scholar] [PubMed]




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.