Prevalence of Chronic Kidney Disease in Iran Army Ground Forces: Correlation between Laboratory Parameters and Demographic Parameters with Kidney Function
2 Department of Nephrology, AJA University of Medical Sciences, Tehran, Iran, Email: Soleimania16@yahoo.com
3 Medical Students Research Center, Isfahan University of Medical Sciences, Isfahan, Iran
Citation: Piri-Ardakani M, et al. Prevalence of Chronic Kidney Disease in Iran Army Ground Forces; Correlation between Laboratory Parameters and Demographic Parameter with Kidney Function. Ann Med Health Sci Res. 2018;8:266-270
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: The prevalence of chronic kidney disease (CKD) is rapidly increasing due to lifestyle changes. Therefore, the current study was aimed to determine the prevalence of CKD in Iran army ground forces. Methods: This cross sectional study was conducted in 2017 on 1,800 health records of the army ground forces of the Islamic Republic of Iran that were referred to four medical centers of the in different cities. Moreover, healthy people over 20 years of age entered the study. Subject with kidney disease and individuals without a complete medical record were excluded. After recording the demographic data and evaluating pathological criteria such as GFR, blood pressure, cholesterol, uric acid, creatinine, proteinuria, FBS and RBC in urine, their relationship with different stages of CKD was then evaluated. Results: subjects consist of 1726 men and 74 women (1800 cases), aged 19–65 years with an average age of 35.47 ± 7. 41 years were included in the study. With regard to the classification, 1597 subjects (89.5%) were assigned in to normal group, followed by 105 subjects with mild stage (5.9%; Stage I), 70 (3.9%) with mild CKD (Stage II) and 11 subjects (1.1%) have moderate CKD (Stage III). After adjusting data, none of the subjects had sever CKD and end stage CKD. Statistical analysis revealed that the age, weight and BMI were significantly higher in patients with CKD (especially Stage I). Moreover, Uric acid, HDL, cholesterol, FBS, Red Blood Cells (RBC) in the urine and also the frequency of proteinuria were significantly higher in patients suffering from CKD (p<0.05). Conclusion: Unhealthy clinical conditions of the body such as blood pressure, blood glucose, cholesterol and high weight, and risk factors such as proteinuria, age, and RBC in the urine are significantly related to CKD.
Keywords
Chronic Kidney Disease (CKD); End-stage renal disease; Prevalence; BloodIntroduction
Changing lifestyle in developing countries has led to an increase in the incidence of non-communicable diseases such as diabetes mellitus, obesity, hypertension, cardiovascular disease and chronic kidney disease (CKD). It has been revealed that deaths caused by chronic diseases highlight significant mortality rates in middle and lower income countries. [1] Among these diseases, chronic kidney disease is one of the most important diseases, because in addition to morbidity and mortality and high prevalence of end-stage renal disease (ESRD) with high societal cost, it is also the most important risk factor for cardiovascular disease. [2] Recent studies have shown that if chronic kidney disease is diagnosed and treated at an early stage, it can prevent or even prevent ESRD and other adverse effects. [3,4] Among the causes of mortality in developing countries, chronic renal disease is now ranked at the nineteenth, which is 82% higher than in 1990’s. Chronic kidney disease is the ninth cause of death in the United States. [5] The prevalence of chronic kidney disease is also increasing worldwide. According to the NHANES report, the prevalence of chronic kidney disease has been determined to be 10% between 1988 and 1994, while this percent reached 13.1% between 1999 and 2004. [6] It is worth noting that kidney disease impose a profound economic burden to care organizations. In addition to morbidity and psychosocial outcomes (such as depression and unemployment), patients need dialysis and kidney transplant facilities, which impose high costs on society? Over the past decade, more than $ 1.1 trillion has been spent on dialysis in the world. [7] However, early diagnosis and treatment in the early stages of a chronic kidney disease can be prevented or even fully treated. The chronic kidney disease in Stages 1, 2, and 3 has no signs, and when the GFR (Glomerular Filtration Rate) is less than 30, the metabolic and endocrine symptoms can be appeared. Therefore, the early diagnosis of chronic kidney disease can be one of the most important public health issues in the community. Staff and military personnel are subject to periodic care. Considering the high cost of kidney therapies such as peritoneal dialysis, hemodialysis, kidney transplantation and the ease of screening period in this population, the importance of screening in the early stages of chronic kidney disease is revealed. Therefore, the present study was aimed to investigate the prevalence of chronic kidney disease in personnel of Iran army ground forces.
Materials and Methods
This cross sectional study was conducted in 2017 on 1,800 health records of Iran army ground forces that had been referred to four health centers in various cities. Moreover, healthy people over the age of 20 years entered the study. Subjects with kidney disease and patients without a complete medical record were also excluded. Participants in this project were excluded from the study if they had acute kidney damage. Overall, after applying the inclusion and exclusion criteria, 1800 participants were finally enrolled in the study. Informed consent was obtained for all research involving participants and enrolled subjects with information about a clinical investigation. Furthermore, they entered the study with full knowledge of the research process. The variables studied included age, BUN, Cr, FBS, total cholesterol, HDL, LDL, TG and Height, Weight, systolic and diastolic blood pressure (SBP/DBP), as well as history of diabetes mellitus and history of hypertension, which raw data were subsequently entered in the IBM SPSS Statistics version 23. Afterwards, the data normalization for all variables was evaluated and appropriate statistics were used based on a status of relative normality. In this study, non-parametric Kruskal- Wallis test and Chi-square tests were applied to analyze the data. Furthermore, the statistical significance (p-value) was considered to be <0.05. With regard to the serum creatinine concentration and age, the quantitative values of Glomerular Filtration Rate (GFR), for all individuals by using the modification of diet in renal disease (MDRD) study equation were calculated as follows:
186 × (serum creatinine [mg/dL])-1.154 × (age [years])-0.203 × 1.212 (if African-American) × 0.742 (if female)
After calculating the GFR based on the values obtained, individuals were classified at the following stages:
a) Normal: (GFR ≥ 90 ml/min/1.73 m2), No symptoms of kidney damage.
b) Stage I: (GFR ≥ 90 ml/min/1.73 m2), Damage to the kidney with normal or elevated GFR plus having one of the following symptoms:
• Blood creatinine or blood urea is higher than normal range.
• Blood or protein in the urine.
• Evidence of renal damage in MRI, CT scan, ultrasound or X-ray.
• Family history of polycystic kidney disease (PKD).
c) Stage II: (GFR = 60-89 ml/min/1.73 m2), determined as mild CKD
d) Stage III: (GFR = 30-59 ml/min/1.73 m2), defined as moderate CKD
e) Stage IV: (GFR = 15-29 ml/min/1.73 m2), defined as severe CKD
f) Stage V: (GFR <15 ml/min/1.73 m2), defined as end stage CKD
Results
A total of 1,800 people, including 1,726 men (95.9%) and 74 women (4.1%), were enrolled in the study. The subjects were generally between the ages of 19 and 65, with an average age of 35.47 years. Moreover, the average weight of patients was determined as 78.21 kg, followed by average height (178.78 cm) and mean BMI (24.5).
Regarding to the classification, 1597 subjects (89.5%) were in the normal group, followed by 105 subjects with mild stage (5.9%; at risk: Stage I), 70 individuals (3.9%) had mild CKD (Stage II) and 11 individuals (1.1%) have moderate CKD (Stage III). Furthermore, there were no data for 17 people. In addition, none of the people had sever CKD and end stage CKD.
With regard to the classification of individuals in these groups, the differences between these classes were studied in some quantitative variables such as age, height, weight, BMI, creatinine, uric acid, systolic blood pressure, etc. In order to select statistical test, the normality of the data was initially evaluated using Kolmogorov Smirnov test (KS) in four stages of CKD. Regarding the K-S test, the One-way test was applied for evaluating data originate from the same distribution (normal). Kruskal-Wallis test (a rank-based nonparametric test) was used to reveal if there are differences between groups of an independent variable, as a comparison of four stages of the CKD.
The mean age of the subjects classified in the four stages of the CKD was calculated and compared with each other. The statistics from this section of the study have shown that an increased lin age is directly related to the severity of the disease. In other words, the age of the subjects increased significantly from normal to moderate CKD stages (p<0.01). The mean age in normal individuals was determined as 35.08 ± 7.29 years, while the mean age of patients with CKD was defined as 46.54 ± 8.43 years.
Mean weight and height were also evaluated as two other important demographic variables in the four stages of CKD. The statistical results obtained by Kruskal-Wallis test and showed that the mean weight in normal people was lower than those with moderate CKD, but subjects with moderate CKD showed a descending course. The findings demonstrated that the differences between all groups were statistically significant (p=0.001). The mean height of subjects indicated a descending trend between normal people and moderate CKD. Differences between different groups are statistically significant in both variables (p=0.023). In other words, it can be concluded that the average height of participants was significantly lower in the severe condition of the disease.
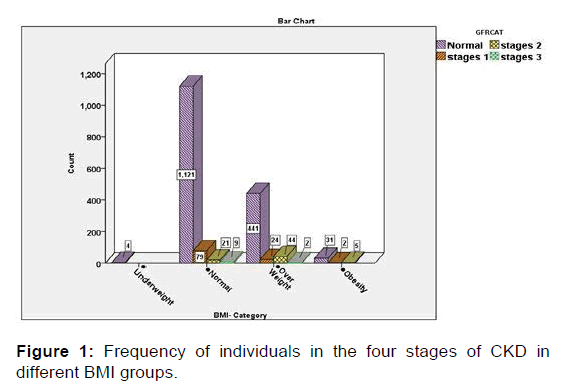
The average BMI, which is an indicator of height and weight, was evaluated in these stages. The results of the Kruskal-Wallis test in accordance with the two main variables (height and weight) revealed that BMI values of the disease in mild CKD was slightly more; however, in patients with severe CKD status, BMI values are at a lower level than other groups. The findings of this test also showed that the difference in mean BMI in the four groups was statistically significant (p=0.001).
Quantitative variable of ureic acid was compared between groups using the Kruskal-Wallis test. Chi-Square test was used to evaluate the frequency distribution of the qualitative variables of proteinuria. The mean of ureic acid among all group indicated that the severity of the disease (from normal to mean CKD) was significantly associated with the increased level of uric acid (p<0.05). Chi-Square test results also revealed a significant relationship between CKD and proteinuria (p=0.001) [Tables 1 and 2].
| Kruskal-Wallis Test | ||||||||
|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | Min | Max | Mean Rank | p-value | ||
| Uric-acid | Normal | 1187 | 6.01 | 1.236 | 0.40 | 14.90 | 639.79 | 0.006 |
| Stage I | 47 | 6.11 | 1.429 | 3.51 | 9.50 | 633.31 | ||
| Stage II | 55 | 6.46 | 1.035 | 3.84 | 8.50 | 804.34 | ||
| Stage III | 5 | 6.76 | 1.087 | 5.60 | 7.90 | 884.9 | ||
| Total | 1294 | 6.04 | 1.238 | 0.40 | 14.90 | ----- | ||
Table 1: Evaluation of uric acid in different CKD stages.
| Proteinuria * GFR Category Cross-tabulation Chi-Square | |||||||
|---|---|---|---|---|---|---|---|
| GFRCAT | Total | p-value | |||||
| Normal | Stage I | Stage II | Stage III | ||||
| Proteinuria | No | 1597 | 0 | 67 | 11 | 1675 | 0.001 |
| Yes | 0 | 105 | 3 | 0 | 108 | ||
| Total | 1597 | 105 | 70 | 11 | 1783 | ||
Table 2: Frequency of proteinuria in four stages of CKD.
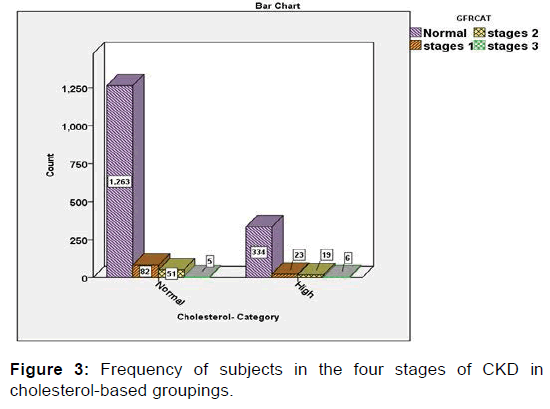
The concentration of lipid profile including HDL, LDL, and triglyceride (TG) was extracted from the subjects and was compared in four stages of CKD. Due to the lack of normalization of data in these variables (based on the K-S test), comparison of mean differences in this group of variables was also performed using Kruskal-Wallis test. The descriptive results of this nonparametric statistical test revealed that the concentrations of the two variables, HDL and LDL, demonstrated incremental trend from stage I to moderate CKD (Stage III), while the TG variable showed a decreasing trend.
The analytical statistics derived from this test determined that only the difference in mean HDL was significant in the four stages of the CKD, and the other variables did not differ significantly in the CKD stages. This means that subjects with different stages of CKD had no significant changes in LDL and TG levels indicating identical trend (p>0.05).
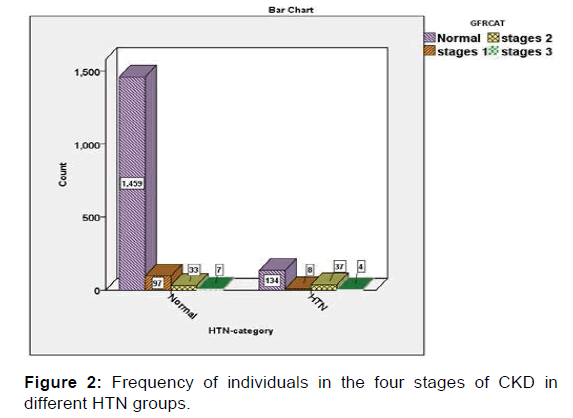
Systolic pressure data was extracted from patients’ files as an important hemodynamic measure and presented as a comparison of CKD stages. The results of the Kruskal-Wallis test indicated that the average blood pressure of the subjects in stage 2 and 3 (mild and moderate CKD) was significantly higher than normal subjects (p=0.001). It is noteworthy that the mean blood pressure in patients with moderate CKD was lower as compared to patients with mild CKD [Table 3].
| Kruskal-Wallis Test | ||||||||
|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | Min | Max | Mean Rank | p-value | ||
| SBP | Normal | 1593 | 119.68 | 10.357 | 90.00 | 160.00 | 872.40 | 0.001 |
| Stage I | 105 | 118.80 | 9.341 | 100.00 | 150.00 | 833.98 | ||
| Stage II | 70 | 133.64 | 15.058 | 105.00 | 160.00 | 1334.46 | ||
| Stage III | 11 | 129.09 | 18.683 | 100.00 | 160.00 | 1145.77 | ||
| Total | 1779 | 120.24 | 10.948 | 90.00 | 160.00 | ----- | ||
Table 3: Comparison of mean systolic blood pressure (SBP) in four stages of CKD.
All subjects were divided into two groups (<140 mmHg, >140 mmHg) for a better examination (based on the American Heart Association), where they were compared with different stages of CKD.
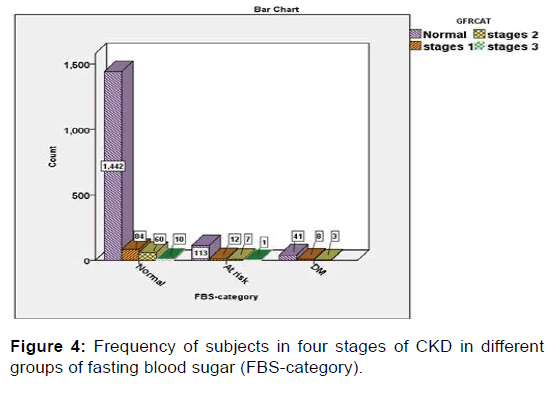
Then, the relationships of BMI, hypertension (HTN), cholesterol, FBS and RBC with CKD were evaluated in the present study. The findings of this part of the study, which was performed using Chi-Square test, revealed that abnormal levels of BMI, blood pressure, and cholesterol, FBS and red blood cells (RBCs) in the urine were significantly associated with a high rate of CKD. In other words, the severity of the condition of the chronic renal disease (CKD) has a significant direct relation with the frequency of abnormal conditions of the five aforementioned variables (p<0.05) [Figures 1-5].
The frequency of proteinuria CKD stages were analyzed by Chi square test. Statistical data demonstrated that there was a statistically significant relationship between sex and proteinuria (p=0.009); however, no correlation was found between sex and CKD. In addition, proteinuria was found in 6.8% of men, while proteinuria was not observed in any of the women (p=0.515).
Discussion
CKD is characterized by a gradual loss of kidney function over time that can be evaluated by GFR. [8]
CKD affects about 47 million adults (14.8%) in the United States and is one of the most important causes of mortality. CKD is associated with a significant increase in the risk of cardiovascular disease and stroke. [9,10] Moreover, cardiovascular disease, family history of CKD, and ethnic minority increase the risk of CKD in individuals over 60 years old suffering from diabetes and high blood pressure (83). GFR is the best marker of kidney function that varies according to age, sex, and body size. [11] Staging the disease is an important step in determining the prognosis, evaluation and treatment of CKD. [12] By applying this type of classification, a powerful tool will be available to physicians who can make valuable health decisions. CKD staging has parameters such as degree of albuminuria, age, sex, and cardiovascular risk factors and other risk factors that can be considered in the treatment process. [13]
The initial examination involves determining the cause and stage of the CKD and assessing the patients. Patient’s history, family history, physical examination, blood pressure, height, weight, blood sugar, blood lipids, and urine output are the most valuable indicators for evaluating CKD. [14] Therefore, laboratory tests should include the measurement of serum glucose and fasting lipid status, ESR, and albumin/creatinine ratio (ACR) or protein-creatinine ratio (PCR). [15] Although it may be necessary to perform more test for determining CKD. Ultrasound may also be needed to assess the existence of possible structural abnormalities.
With regard to available indicators, the occurrence of CKD can be recognized with appropriate confidence by examining and determining the relationship between existing indicators, therefore, therapeutic and supportive measures can be performed to prevent the progression of the disease in the shortest time. Due to health policies, descriptive information, such as the rate and distribution of CKD is needed. So far, there have been no reports in terms of the CKD prevalence and kidney failure among military personnel in Iran. Therefore, the present study was aimed to determine the prevalence of CKD based on the use of laboratory parameters and GFR.
The results of our study showed that there was a significantly higher incidence of advanced CKD in older adults. The average weights in normal people were lower than those with CKD, but people with moderate CKD, experienced a downward trend. A significant association was found in terms of weight between different groups. The mean height of the subjects from the high risk to moderate CKD showed a descending trend, where the difference between the two groups was statistically significant.
BMI in mild CKD group was found to be higher than normal subjects, but BMI was determined at a lower level in subjects suffering from severe CKD. Furthermore, the difference in mean BMI was statistically significant in the four groups. The statistics also revealed that the three variables of creatinine, uric acid and proteinuria increased significantly with the severity of the disease (from normal to moderate CKD).
The concentration of HDL and LDL in patients with Stage 1 and those with moderate CKD (Stage 3) showed an increasing trend. Nevertheless, in the TG variable, this trend had decreased. Statistical analyzes indicated that the mean of HDL was significantly difference among four stages of CKD and there was no significant difference in terms of other two variables.
The mean of blood pressure in stages II, III was significantly higher than mild CKD. In addition, people who have BMI, hypertension, cholesterol, FBS and RBC in abnormal levels are more likely to have mild and severe CKD. There was a statistically significant relationship between sex and proteinuria (all cases of proteinuria were male), but there was no significant relationship between sex and CKD.
Over the past years, many efforts have been made by many researchers to explain various aspects of the subject. Each research team has focused on the subject and reported their results. Lash et al. conducted a chronic renal insufficiency cohort (CRIC) investigation to evaluate risk factors for the progression of CKD.
They reported that lower level of eGFR could be linekd to a higher burden of CVD, as well as lower level of education and socioeconomic status. Forty seven percent had diabetes and 47% had hypertension (>130 mmHg). The mean BMI in these subjects was 32.1 ± 7.9 and the mean GFR was 43.4 ± 13.5. [16]
The results of this study are consistent with the study, but there are also some quantitative differences. For example, in our study, 26.3% of subjects with different degrees of CKD had hypertension at the same time, and only about 6% of CKD patients had diabetes, it was also found that individuals with stage 3 had an average GFR equivalent to 53/ml/min/1.73 m2 and the maximum BMI of subjects (26.32 kg/m2) was mostly observed in moderate CKD, all of which had numerical values less than those of Lash and colleagues.
Another study by Viktorsdottir et al. determined eGFR based on the use of serum creatinine for estimating the prevalence of CKD in the Icelandic population. The participants of aforementioned study had consisted of 19256 individuals (9229 mean and 10 027 women), aged 33–85 years. They demonstrated that more than 50% of subjects had an eGFR in the range of 60–89 ml/min/1.73 m2. While in the present study, less than 10% of the subjects had GFR less than 89.7 ml/min/1.73 m2. [17]
They also showed that age-associated prevalence of low eGFR was determined as 4.7 and 11.6% for males and females (subjects aged 35–80+ years). Moreover, 93.2% of men and 89.9% of women had proteinuria, which are contradictory with our study. Because we found thatthe prevalence of CKD in men and women was 10.5% and 9.9%, respectively and the prevalence of proteinuria in men and women was determined to be 6.4% and 0%, respectively. In the current study, the eGFR <60 score increased with age, which is in agreement with mentioned study. This should be taken into account that dyslipidemia itself is a risk factor for CVD. Due to complications and cardiovascular morbidity and mortality of patients with CKD, extensive studies have been conducted with sometimes-contradictory results. This shows the importance of examining lipid metabolism in patients with CKD.
Zubovic et al. conducted a one-year prospective study to lipid status of patients with different stages of CKD. The participants of consisted of 71 males and 79 females (150 adult patients). They reported that the highest serum cholesterol levels were determined in patients with stage II renal disease and the lowest in stage IV. Furthermore, they revealed that the level of cholesterol did not significantly correlate with the progression of CKD. Analysis of the average value of triglyceride in their study also showed that, there was a slight increase in this variable by increasing CKD stages, so that the lowest serum triglyceride levels were observed in stage I (1.73 ± 1.17 mmol/L), while the highest values was determined in Stage III (2.13 ± 1.11 mmol/L). However, these increase values were not statistically significant. [18] Yanni Wang et al. found that TG level had a negative correlation with CKD, but the difference was not significant between CKD stages. Furthermore, their results showed that HDL had a non-significant reduction in CKD stages, and LDL only has a non-significant increase in Stage II. In overall they suggested that TG level is independently linked to mGFR. [19]
Overall, the results of the studies show remarkable differences in detail as compared with some previous studies. Nevertheless, with a comprehensive look at the outcomes of this study, it will be important to note that, although there are ambiguities and controversies in some issues, there is nevertheless a comprehensive agreement between this research and other studies.
There are significant differences in scientific literature, especially in the prevalence of CKD. The main reasons for this difference are factors such as sample size and targeted community, age, gender, demographic characteristics, different ethnicities, technical factors and technological differences (for measuring small amounts of variables), as well as inclusion and exclusion criteria.
Conclusion
Considering the significant relationships between some clinical symptoms with CKD, it can be concluded that having a healthy lifestyle and a good diet can be control factors involved in CKD, such as blood pressure, blood sugar, cholesterol and high weight.
Acknowledgement
The research team appreciates the older adults and AJA University of Medical Sciences personnel who helped us in conducting this research.
Conflicts of Interest
The authors disclose that they have no conflicts of interest.
REFERENCES
- Stel VS, Brück K, Fraser S, Zoccali C, Massy ZA, Jager KJ. International differences in chronic kidney disease prevalence: a key public health and epidemiologic research issue. Nephrol Dial Transplant 2017;32:II129-II135.
- Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Annals of Internal Medicine 2001;134:629-636
- Eknoyan G, Levey AS, Levin NW, Keane WF. The national epidemic of chronic kidney disease: What we know and what we can do. Postgraduate Medicine 2001;110:23-29.
- Goolsby MJ. National Kidney Foundation Guidelines for chronic kidney disease: Evaluation, classification, and stratification. Journal of the American Association of Nurse Practitioners 2002;14:238-242.
- Peterson CB. Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;385:117-171.
- Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007;298:2038-2047.
- Lysaght MJ. Maintenance dialysis population dynamics: Current trends and long-term implications. Journal of the American Society of Nephrology 2002;13:S37-S40.
- Baumgarten M, Gehr T. Chronic kidney disease. Detection and evaluation. Am Fam Physician. 2017;96:776-783.
- Collins AJ, Li S, Gilbertson DT, Liu J, Chen SC, Herzog CA. Chronic kidney disease and cardiovascular disease in the Medicare population: Management of comorbidities in kidney disease in the 21st century: Anemia and bone disease. Kidney International 2003;64:S24-S31.
- Gaitonde DY, Cook DL, Rivera IM. Chronic kidney disease: Detection and evaluation. Am Fam Physician. 2017;96:776-783.
- Levey AS, Coresh J, Bolton K, Culleton B, Harvey KS, Ikizler TA, et al. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1-266.
- Qaseem A, Hopkins RH, Sweet DE, Starkey M, Shekelle P. Screening, monitoring, and treatment of stage 1 to 3 chronic kidney disease: A clinical practice guideline from the American College of Physicians. Annals of Internal Medicine. 2013;159:835-847.
- Levey AS, Becker C, Inker LA. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: A systematic review. JAMA 2015;313:837-846.
- Johnson DW, Atai E, Chan M, Phoon RK, Scott C, Toussaint ND, et al. KHA- Guideline: early chronic kidney disease: Detection, prevention and management. Nephrology 2013;18:340-350.
- Kerr M, Bray B, Medcalf J, O'donoghue DJ, Matthews B. Estimating the financial cost of chronic kidney disease to the NHS in England. Nephrology Dialysis Transplantation. 2012;27:III73-III80.
- Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, et al. Chronic Renal Insufficiency Cohort (CRIC) Study: Baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4:1302-1311.
- Viktorsdottir O, Palsson R, Andresdottir MB, Aspelund T, Gudnason V, Indridason OS. Prevalence of chronic kidney disease based on estimated glomerular filtration rate and proteinuria in Icelandic adults. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2005;20:1799-1807.
- Zubovic SV, Kristic S, Prevljak S, Pasic IS. Chronic kidney disease and lipid disorders. Medical Archives.2016;70:191-192.
- Wang Y, Qiu X, Lv L, Wang C, Ye Z, Li S, et al. Correlation between serum lipid levels and measured glomerular filtration rate in Chinese patients with chronic kidney disease. PLOS ONE 2016;11:e0163767.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.