Prevalence of Fungal, Bacterial, and Viral Sexually Transmitted Diseases as Well as Monthly Trends in Tigray, Ethiopia's Government Health Facilities, July 2019-June 2020
2 Department of Biological and Chemical Engineering, Mekelle Institute of Technology, Mekelle University, Ethiopia
Received: 04-Mar-2024, Manuscript No. AMHSR-24-128793; Editor assigned: 06-Mar-2024, Pre QC No. AMHSR-24-128793 (PQ); Reviewed: 20-Mar-2024 QC No. AMHSR-24-128793; Revised: 10-Mar-2025, Manuscript No. AMHSR-24-128793 (R); Published: 17-Mar-2025, DOI: 10.54608.annalsmedical.2025.185
Citation: Gebremedhin MT, et al.. Prevalence of Fungal, Bacterial, and Viral Sexually Transmitted Diseases As Well As Monthly Trends in Tigray, Ethiopia's Government Health Facilities, July 2019-June 2020. Ann Med Health Sci Res. 2025;15:1-7
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Objective of the study: To assess the prevalence and trends of bacterial, fungal and viral STIs in our region.
Methods: A retrospective study on Sexually Transmitted Diseases (STDs) in healthcare facilities in the Tigray regions was carried out between July 2019 and June 2020. Descriptive statistics analysis was utilized to compare prevalence measures and trends across age groups, sexes, and months. Tables and line graphs were used to present the indings. Using the Chi-square test, a relationship between the disease conditions and was found.
Main outcome measures: Measures of prevalence and trend.
Result: 3500 patients who visited the hospital are included in this analysis. Of those who received a diagnosis, 1657 (47.3%) were in females and 1843 (52.7%) in males. When it came to STIs spread by viruses, males were more affected than females among these individuals, but females were more affected than males when STIs were spread by bacterial and fungal infections. Hepatitis B (16.9%), gonorrhea (16.3%), hepatitis C (13.6%), syphilis (11.8%), genital herpes (8.8%), genital warts (7.5%), and chlamydia (6.8%) are the conditions that affect the genital area. The pattern of sexual transmission was intermittent.
Conclusion: Adults are the main target of sexually transmitted infections, which vary in frequency depending on gender and age. The results provide information about the epidemiological state of the nation and suggest future preventative measures. Reducing the prevalence of STDs requires concentrating interventions on high-risk individuals in the 14-29 age range. Consequently, it is advised to create and fortify reproductive health centers on campuses, spread awareness of sexual and reproductive health issues, including STI modes of transmission, prevention, and health-seeking behaviors, as well as to improve condom availability and information.
Keywords
Sexual transmitted disease; Prevalence; Trend; Governmental health facility
Abbreviations
AS: Age-Standardized; CT: Chlamydia Trachomatis; DHIS: District Health Information System; EAPC: Estimated Annual Percentage Change; GBDS: Global Burden of Disease Study; HFs: Health Facilities; HIV: Human Immunodeficiency; HMIS: Health Resource Information System; MSM: Men who have Sex with Men; PID: Pelvic Inflammatory Disease; SDI: Socio Demographic Index; STIs: Sexually Transmitted Infections; TRHB: Tigray Regional Health Bureau; WHO: World Health Organization
Introduction
Globally, STIs continue to have a major detrimental influence on public and social health, especially in developing countries [1]. Sexually Transmitted Infections (STIs) represent a significant health and economic burden for developing nations: These nations account for 75–85% of the estimated 340 million new cases of treatable STIs each year, and STIs generate 17% of economic losses due to illness [2]. Developing nations are facing a number of public health challenges due to genital tract infections [3]. Social stigma, in particular, has a significant impact on STI preventive behaviors and necessitates effective intervention [4].
Sexually Transmitted Infections (STIs) have direct impact on sexual and reproductive health through stigmatization, infertility, cancers and pregnancy complications [5]. However, in addition to a lack of understanding about STDs and the services that are available, a number of significant obstacles to receiving care were found. In addition, out-of-pocket expenses were a major deterrent to getting medical attention [6]. Untreated Sexually Transmitted Diseases (STDs) have been linked to complications such as adverse pregnancy and perinatal outcomes [7]. Screening for sexually transmitted infections in women is crucial for detecting asymptomatic infections before complications develop and reducing their recurrence, according to studies [8].
Limited resources in developing countries lead to syndrome treatment for STDs. Social stigma, weak health systems, funding, and policy support hinder STI prevention. Sub-Saharan Africa has high rates of syphilis, with a 20% increase between 2006 and 2007, posing a public health problem, particularly in low-income countries [9]-[15]. Two systematic reviews of STI estimates from 2012 and 2016 revealed high prevalence and incidence of chlamydia, gonorrhea, and syphilis in adults, with nearly a million new treatable diseases occurring daily, but the number varied by region and gender [16]. There is an attempt to estimate the burden of many diseases according to age, gender, and geographical location in different parts of the world from 1990 to 2019 [17]. The WHO global health strategy on sexually transmitted infections 2016-2021 aims to gather data on the prevalence and incidence of sexually transmitted diseases in representative populations [18].
Progress has been made in the development and introduction of vaccines against certain sexually transmitted diseases such as hepatitis B and human papillomavirus. The development of a rapid point-of-care test for syphilis has opened the door to controlling this infection [19]. Despite extensive studies on preventing sexually transmitted diseases in adolescents, there is inconsistent prevalence data, and previous studies may not accurately represent the overall trend and prevalence of these diseases was not show the overall health facility and limited data was available in Ethiopia that show overall picture of all health facility in our region Tigray Ethiopia. Therefore, the aim of this review was to determine the prevalence and trend of sexually transmitted diseases in the patients visiting health facility in Tigray. Understanding the prevalence and trends of STDs in our region is crucial for developing targeted interventions and this also improving public health effort to reduce the burden of STDs in our community.
Materials and Methods
Study design
This study was a retrospective data analysis; assessing prevalence and estimated monthly percentage change was calculated to quantify trends in age-standardized prevalence, stratified by age and sex of communicable infection transmitted by sexual contact among screened patients at all health facilities in from July 2019 to June 2020 using data from a monthly reporting system comprising data from all health facilities in the region. Sexual transmitted infection screening for all people visiting health facilities, consisting of assessing signs and symptoms through interrogation and/or all in each health facility. The data stored in regional health from all health included screening data. Data of high quality according to studies evaluating the quality of data conducted in 2019-2020, where high proportions of health facilities achieved acceptable factors for data on different indicators in all districts.
Study population and period
All patients attending the health facility syndromatic diagnosis with STI disease at the health facility between July 2019 and June 2020 were included.
Data collection procedure
Numbers are electronically recorded in Health management information system. Recorded in Excel from the health system database collection form. Collected data, number of patients screened, and years. Data was from the region-wide screening of the 2019–2020 period. The study outcomes of interest were the number of screened disease transmitted by sexual contact. The region is organized into seven zones with 94 provinces being totally composed of 10 urban districts, while other provinces are composed of at least 1 or 2 semiurban districts and rural districts. Its health system is into public and private health facilities. The overall health service coverage of the region has reached 90%, having one specialized referral hospital, 16 hospitals, 22 primary hospitals, and 224 health centers owned by the government.
Eligibility criteria
All patients over the age of one who visited STI clinics throughout the region and whose diagnostic results were completely captured in the HMIS data set. Our investigation excluded patient recordings having a degraded record or missing data.
Data analysis
Data entry in to Excel version 10 and exported to SPSS version 14 for analyses. A descriptive analysis was performed and presented in frequencies and proportions using tables and figures.
Results
The study includes diseases transmitted by sexual contact. Of the diagnosed, 1657 were diagnosed in females (47.3%) and 1843 were diagnosed in males (52.7%). Males reported significantly more affected than females.
The prevalence of gonococci infection was found in 61.4% of women and 38.6% of men, with female adults making up the majority. The age range of 14 to 29 years old showed the greatest increase in gonococci prevalence. The prevalence of genital candidiasis was found in 40.1% of men and 59.9% of women, with adult females aged 14 to 29 making up the majority. The 95% confidence interval for this disease was 18.3%. Syphilis prevalence was 11.8% (95% CI: 10.7–13.0). 62.2% of adult females versus 37.8% of male adults aged 30-34 infected with syphilis. The percentage of adults aged 30-34 who had Chlamydia infection was 6.8% 95% CI (6.0-7.6), 64.6% in women, and 35.4% in men. Adult females accounted for the majority of cases (Table 1).
| Variable | Prevalence | Female | <14 years | 14-29 years | 30-34 years | >=35 year | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| N(%) | N(%) | N(%) | N(%) | N(%) | N(%) | N(%) | ||
| Chlamydia | 237 (6.8) | 84 (35.4) | 153 (64.6) | 17 (7.2) | 82 (34.6) | 117 (49.4) | 21 (8.9) | (6-7.6) |
| Syphilis | 413 (11.8) | 156 (37.8) | 257 (62.2) | 8 (1.9) | 184 (44.6) | 194 (47) | 27 (21.7) | (10.7-13) |
| Gonococci | 572 (16.3) | 351 (61.4) | 221 (38.6) | 142 (24.8) | 211 (36.9) | 124 (21.7) | 95 (16.6) | (15.1-17.7) |
| Genetal candidias | 641 (18.3) | 257 (40.1) | 384 (59.9) | 185 (28.9) | 247 (38.5) | 183 (28.5) | 26 (4.1) | (17-19.6) |
Table 1: Prevalence of bacterial and fungal sexual transmitted disease by age and gender.
Genital herpes prevalence was 8.8% 95% CI (7.9-9.6), with 53.4% of men and 46.6% of women having the highest age-related increase in herpes prevalence between the ages of 14 and 29. Genital wart prevalence was 7.4% 95% CI (6.6-8.3), and it rose most with age in the 30-34 age group. 39.8% vs. 60.2% male. The prevalence of hepatitis C was 33.9% in females and 13.6% 95% CI (12.5-14.8) in men, with the adult male population primarily aged 30-34. 18.3% of people had hepatitis B (15.7–18.3). It has been demonstrated that adults had a higher prevalence than children, with adult males having a higher percentage than children (30.9% vs. 60.6% males) (Table 2).
| Variable | Prevalence | Male | Female | <14 years | 14-29 years | 30-34 years | >=35 years | 95% CI |
|---|---|---|---|---|---|---|---|---|
| N(%) | N(%) | N(%) | N(%) | N(%) | N(%) | N(%) | ||
| Genital herpes 2 | 307 (8.8) | 164 (53.4) | 143 (46.6) | 58 (18.9) | 120 (39.1) | 107 (34.9) | 22 (7.2) | (7.9-9.6) |
| Hepatitis B | 591 (16.8) | 358 (60.6) | 233 (39.4) | 146 (24.7) | 124 (21) | 230 (38.9) | 91 (15.4) | (15.7-18.3) |
| Genetal warts | 261 (7.4) | 157 (60.2) | 104 (39.8) | 37 (14.2) | 144 (56.2) | 72 (27.6) | 8 (3.1) | (6.6-8.3) |
| Hepatitis C | 478 (13.6) | 316 (66.1) | 162 (33.9) | 10 (2.1) | 117 (24.5) | 294 (61.5) | 57 (11.9) | (12.5-14.8) |
Table 2: Prevalence of viral sexual transmitted disease by age and gender.
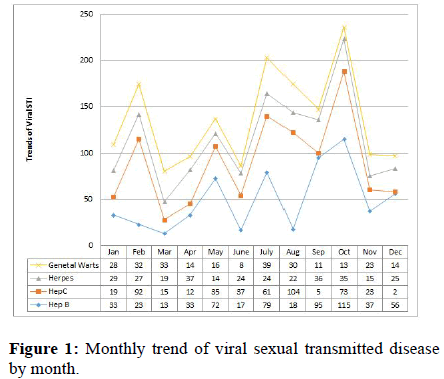
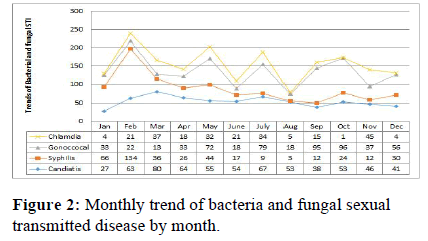
Prevalence of hepatitis B was highest in October whereas lowest in December 2020. Decline rates for sexually transmitted infections were highest between February and March and decreased from October to December for hepatitis B virus, with higher rates in August for both hepatitis C and genital warts. The decreasing trends in sexually transmitted infection prevalence observed in this study over the period, by the end of November 2020 there was a significant decrease in hepatitis B virus cases in November, hepatitis C cases in September, genital herpes cases in May, genital wart cases in September (Figure 2). There was significant difference between gender and age group with the prevalence of sexual transmitted infection (and trend and statistically trend for a decrease in the prevalence of most sexual transmitted infection. In general, the prevalence line in the graph showed that the prevalence of viral sexually transmitted infection in the region was intermittent in one year from July 2019 to June 2020 in Figure 1.
The highest disease transmitted by sexual contact was observed in February with a syphilis case. Lowest transmitted occurred in October with a chlamydia case. Decrease in sexual transmitted infection was occurred between July and August. Increased from March to August for genital candida, February to March for syphilis, October to November for gonococci, and November for chlamydia. syphilis increases when compared to the base line at the end of the year.
Decreasing in syphilis prevalence observed in this study over the one-year period (September to June 2020) was followed by a sharp decrease in genital candidasis (December), chlymidia (November), gonococci (May), and genital warts (September) in the region. The monthly decreased in STI was not statistically significant and in general, STI by gender indicated that women were less likely to have STI than men. In general, the prevalence line in the graph (Figure 2) showed that the extent of bacterial and fungal STIs in the region is intermittent in one year from July 2019 to June 2020. The prevalence of syphilis cases increased from 2.2 to 4.1% in 2019-2020 (Figure 2).
Discussion
The aim of this study was to assess sexual transmitted disease in the general population attending various health facilities from July 2019 to June 2020 by age and sex categories based on a one-year database.
The findings of our investigation revealed a comparatively high frequency of STDs in this population: Genital herpes (8.8%), genital warts (7.5%), syphilis (11.8%), hepatitis B (16.9%), gonococci (16.3%), hepatitis C (13.6%), and Chlamydia infection (6.8%). According to the study, the prevalence of STS decreased from 2019 to 2020 over a one-year period, with adult patients being more affected than pediatric patients. While hepatitis, Chlamydia, and syphilis infections were more common in the 30 to 34-year-old age range, the majority of sexually transmitted viral infections were found in the 14–29 age groups.
Prevalence of hepatitis B and hepatitis C was 16.9% and 13.6%, respectively. Which is higher than the result found in Ethiopia, hepatitis B and hepatitis C virus was found to be 3.57% and 2.13 respectively [20]. It might be because the study was done in a single hospital, the sample sizes were different, and the data analysis was done using six years of data. Our result was lower than the results reported in Europe, 23.4% for hepatitis B and 31.2% for hepatitis C virus [21]. This variation may result from the study population and the use of multiple laboratory tests to confirm the hepatitis, which could raise the study's hepatitis prevalence. In this study, the more males were affected. This is similar to Kazakhstan report [22].
Prevalence of genital herpes was 8.8%. It agrees with the finding from Italy [23]. And also lower than report from Brazil, herpes 15.6% [24]. This discrepancy may be due to the study population and area; both sex was involved and the sample size was larger than in Brazil, where the only participants in Brazil were women. Our result was higher than from Fingerâ?Jardim F, et al. report [25]. The study's findings varied in prevalence rates, which may have been caused by the fact that only pregnant women participated in the research, which was carried out in Ethiopia, and that the disease was diagnosed using PCR, which overestimates the results in this study.
Genital warts were 7.4% which is higher than in India and was estimated at 1.07% [26], this result was lower than result reported from Iran it was 9.2% [27]. This discrepancy was caused by variations in study durations and sample sizes. This result aligned with the sub-Saharan range of 3.5% to 10.5% [28].
The prevalence of Chlamydia infection in this study was 6.8% which is lower than sub-Saharan Africa is 7.8% [29]. This is expected the pooled mean prevalence was more accurately determined by meta-analysis than by this one study. This study was found to be higher than the Tanzania result found (4.6%) [30]. This discrepancy may result from variations in the study population, diagnosis technique, and study setting.
Prevalence of syphilis in this research was 11.8%, which was higher than the study conducted in in China, 6.3% [31]. Our results were in line with the Ghatemala range [25]. The findings from Brazil were found to be lower [32]. Due more vulnerable study population involved in the study and sample size.
Prevalence of gonococci was 16.3%. It was higher than what was found in places like sub-Saharan Africa 7.6% in sex workers [33], Tanzania, it was 11.4% [34]. This difference may be due to study population and sample size. Our result was within the Europe gonococcus range. It was 0-18.5% [35]. This finding was differ with these finding it might be due to diagnosis method used and study population over estimate our result.
The study revealed that 18.3% of individuals had genital candidacies, a higher rate than the findings of Odysseus Gregarious, et al. [36], but lower than the rate reported in Lebanon [37]. This differs due study population and socioeconomic variations.
This study found inconsistent trends in the prevalence of sexual transmitted diseases, similar to the found result in South Ethiopia [38]. The prevalence of Sexually Transmitted Diseases (STDs) in sub-Saharan Africa is decreasing [38]. Our research indicates that the majority of Sexually Transmitted Diseases (STDs) occur in individuals aged 15-29, consistent with global studies [39].
In general our study found that STDs trend was irregular, similar to a study in Ethiopia [40]. The prevalence results were higher than in Ethiopia, East and sub-Saharan Africa, but lower than in Europe, Egypt and Brazil.
Strength and weakness
The ability to collect and aggregate data from a large number of patients is the strength of the retrospective review; the availability of some missing data is its weakness. Additionally, this study focused on the most treatable STDs rather than including all STDs.
Conclusion
Sexual transmitted diseases were widespread in this region, and affected people of all ages and genders. STI can have a more pronounced impact on disease burden at the individual and population levels. Understanding the trend and prevalence of STI diseases may help to implement of regionally based allocation resource for STI prevention aimed at lowering STI rates and early identify persons at highest risk for STIs and to offer prevention services to reduce their risk of acquisition and transmission, especially to highly stigmatized populations.
Author Contributions
MG AT conceived the study and designed the protocol and performed analyses of disease from the database, drafted the manuscript. MG, GT, AT, and MZ, GG detailed write-up and critically revising the manuscript. This work was carried out in collaboration between all authors. All authors have read and reviewed the content of the final manuscript and have approved the manuscript for submission.
Acknowledgments
The authors would like to thank Tigray health bureau for provision of all the HMIS data set and Tigray Health Research Institute. Moreover, the authors would like to acknowledge to all staffs particularly those who are working in the HMIS data encoding.
Funding
No fund received for this study because of retrospective study.
Availability of Data and Materials
The datasets generated and/or analyzed during the current study are not publicly available because of the sensitive nature of the data but are accessible from the corresponding author at a reasonable request.
Competing Interests
The authors declare that they have no competing interests relevant to this manuscript.
References
- Caruso G, Giammanco A, Virruso R, Fasciana T. Current and future trends in the laboratory diagnosis of sexually transmitted infections. Int J Environ Res Public Health. 2021;18:1038.
[Crossref] [Google Scholar] [PubMed]
- Mayaud P, Mabey D. Approaches to the control of sexually transmitted infections in developing countries: old problems and modern challenges. Sex Transm Infect. 2004;80:174.
[Crossref] [Google Scholar] [PubMed]
- Yasin J, Ayalew G, Dagnaw M, Shiferaw G, et al. Vulvovaginitis prevalence among women in gondar, Northwest Ethiopia: Special emphasis on aerobic vaginitis causing bacterial profile, antimicrobial susceptibility pattern, and associated factors. Infect Drug Resist. 2021:4567-80.
[Crossref] [Google Scholar] [PubMed]
- de Wit JB, Adam PC, Den Daas C, Jonas K. Sexually transmitted infection prevention behaviours: health impact, prevalence, correlates, and interventions. Psychol Health. 2023;38:675-700.
[Crossref] [Google Scholar] [PubMed]
- Rao CR, Rao R. Impact of Sexually Transmitted Diseases on Public Health. Sex Transm Oral Dis. 2023:37-42.
- Tilson EC, Sanchez V, Ford CL, Smurzynski M, et al. Barriers to asymptomatic screening and other STD services for adolescents and young adults: focus group discussions. BMC Public Health. 2004;4:1-8.
[Crossref] [Google Scholar] [PubMed]
- Johnson HL, Ghanem KG, Zenilman JM, Erbelding EJ. Sexually transmitted infections and adverse pregnancy outcomes among women attending inner city public sexually transmitted diseases clinics. Sex Transm Dis. 2011;38:167-171.
[Crossref] [Google Scholar] [PubMed]
- Gottlieb SL, Xu F, Brunham RC. Screening and treating Chlamydia trachomatis genital infection to prevent pelvic inflammatory disease: interpretation of findings from randomized controlled trials. Sex Transm Dis. 2013;40:97-102.
[Crossref] [Google Scholar] [PubMed]
- Chanakira E, O’Cathain A, Goyder EC, Freeman JV. Factors perceived to influence risky sexual behaviours among university students in the United Kingdom: a qualitative telephone interview study. BMC Public Health. 2014;14:1-7.
[Crossref] [Google Scholar] [PubMed]
- Chen M, Liao Y, Liu J, Fang W, et al. Comparison of sexual knowledge, attitude, and behavior between female Chinese college students from urban areas and rural areas: a hidden challenge for HIV/AIDS control in China. Biomed Res Int. 2016.
[Crossref] [Google Scholar] [PubMed]
- Goundry AL, Finlay ER, Llewellyn CD. Talking about links between sexually transmitted infections and infertility with college and university students from SE England, UK: a qualitative study. Reprod Health. 2013;10:1-7.
[Crossref] [Google Scholar] [PubMed]
- Hong ZH, Wang XY, Fang YE, Gu HH, et al. Contraceptive knowledge, attitudes and behavior about sexuality among college students in Beijing, China. Chin Med J. 2012;125:1153-1157.
[Google Scholar] [PubMed]
- Ma W, Chen Z, Niu S. Advances and challenges in sexually transmitted infections prevention among men who have sex with men in Asia. Curr Opin Infect Dis. 2023;36:26-34.
[Crossref] [Google Scholar] [PubMed]
- Nigussie T, Yosef T. Knowledge of sexually transmitted infections and its associated factors among polytechnic college students in Southwest Ethiopia. Pan Afr Med J. 2020;37:68.
[Crossref] [Google Scholar] [PubMed]
- Al Ramahi M, Mahafzah A, Saleh S, Fram K. Prevalence of Chlamydia trachomatis infection in infertile women at a university hospital in Jordan. EMHJ-Eastern Mediterr Health J. 2008;14:1148-1154.
- Rowley JV, Vander Hoorn S, Korenromp E, Low N, et al. Global and regional estimates of the prevalence and incidence of four curable sexually transmitted infections in 2016. WHO Bull. 2019;97:548-562.
- AlMukdad S, Harfouche M, Farooqui US, Aldos L, et al. Epidemiology of herpes simplex virus type 1 and genital herpes in Australia and New Zealand: systematic review, meta-analyses and meta-regressions. Epidemiol Infect. 2023;151:e33.
[Crossref] [Google Scholar] [PubMed]
- Organization WH. Global Health Sector Strategy on Sexually Transmitted Infections 2016–2021. Geneva: World Health Organization (2016). [Crossref] [Google Scholar] [PubMed]
- Ortayli N, Ringheim K, Collins L, Sladden T. Sexually transmitted infections: progress and challenges since the 1994 International Conference on Population and Development (ICPD). Contraception. 2014;90:S22-S31.
[Crossref] [Google Scholar] [PubMed]
- Legese H, Berhe B, Adhanom G, Kahsay T, et al. Trend analysis of hepatitis B and C among patients visiting health facility of Tigrai, Ethiopia, 2014–2019. BMC Gastroenterol. 2023;23:164.
[Crossref] [Google Scholar] [PubMed]
- Saaed FM, Ongerth JE. Prevalence of Hepatitis B and Hepatitis C in Migrants from Sub-Saharan Africa Before Onward Dispersal Toward Europe. J Immigr Minor Health. 2023;25:882-888.
[Crossref] [Google Scholar] [PubMed]
- Jumabayeva A, Nersesov A, Kulzhanov M, Nefedova M, et al. Prevalence of viral Hepatitis B, C, and D in Kazakhstan. ScientificWorldJournal. 2022.
[Crossref] [Google Scholar] [PubMed]
- Marchi S, Trombetta CM, Gasparini R, Temperton N, et al. Epidemiology of herpes simplex virus type 1 and 2 in Italy: a seroprevalence study from 2000 to 2014. J Prev Med Hyg. 2017;58:E27.
[Google Scholar] [PubMed]
- Caldeira TD, Goncalves CV, Oliveira GR, Fonseca TV, et al. Prevalence of herpes simplex virus type 2 and risk factors associated with this infection in women in southern Brazil. Rev Inst Med Trop Sao Paulo. 2013 Sep;55:315-21.
[Crossref] [Google Scholar] [PubMed]
- Fingerâ?Jardim F, Avila EC, da Hora VP, Goncalves CV, et al. Prevalence of herpes simplex virus types 1 and 2 at maternal and fetal sides of the placenta in asymptomatic pregnant women. Am J Reprod Immunol. 2017;78:e12689.
[Crossref] [Google Scholar] [PubMed]
- Khopkar US, Rajagopalan M, Chauhan AR, Kothari-Talwar S, et al. Prevalence and burden related to genital warts in India. Viral Immunol. 2018;31:346-351.
[Crossref] [Google Scholar] [PubMed]
- Faridizad R, Alavi A, Golshiri P, Alavi Shoushtari SM, et al. Factors Associated with Anogenital Warts and Gonorrhea Infection: A Cross-Sectional Study. J Health Sci Surveill Syst. 2022;10:113-118.
- Banura C, Mirembe FM, Orem J, Mbonye AK, et al. Prevalence, incidence and risk factors for anogenital warts in Sub Saharan Africa: a systematic review and meta-analysis. Infect Agent Cancer. 2013;8:1-10.
[Crossref] [Google Scholar] [PubMed]
- Hussen S, Wachamo D, Yohannes Z, Tadesse E. Prevalence of chlamydia trachomatis infection among reproductive age women in sub Saharan Africa: a systematic review and meta-analysis. BMC Infect Dis. 2018;18:1-8.
[Crossref] [Google Scholar] [PubMed]
- Juliana NC, Deb S, Ouburg S, Chauhan A, et al. The prevalence of chlamydia trachomatis and three other non-viral sexually transmitted infections among pregnant women in Pemba Island Tanzania. Pathogens. 2020;9:625.
[Crossref] [Google Scholar] [PubMed]
- Jin Z, Wang D, Zhang H, Liang J, et al. Incidence trend of five common musculoskeletal disorders from 1990 to 2017 at the global, regional and national level: results from the global burden of disease study 2017. Ann Rheum Dis. 2020;79:1014-1022.
[Crossref] [Google Scholar] [PubMed]
- Azevedo Junior WS, Santos EP, Pedreira NP, Dantas LB, et al. Prevalence and vulnerability factors associated with HIV and syphilis in older people from subnormal agglomerate, Brazilian Amazon. Trop Med Infect Dis. 2022;7:332.
[Crossref] [Google Scholar] [PubMed]
- Dubbink JH, Verweij SP, Struthers HE, Ouburg S, et al. Genital Chlamydia trachomatis and Neisseria gonorrhoeae infections among women in sub-Saharan Africa: A structured review. Int J STD AIDS. 2018;29:806-824.
[Crossref] [Google Scholar] [PubMed]
- Mayaud P, Cornelissen J, Todd J, Kaatano G, et al. Validation of a WHO algorithm with risk assessment for the clinical management of vaginal discharge in Mwanza, Tanzania. Sex Transm Infect. 1998;74:S77-S84.
[Google Scholar] [PubMed]
- Barbaric J, Kuchukhidze G, Seguy N, Vovc E, et al. Surveillance and epidemiology of syphilis, gonorrhoea and chlamydia in the non-European Union countries of the World Health Organization European Region, 2015 to 2020. Euro Surveill. 2022;27:2100197.
[Crossref] [Google Scholar] [PubMed]
- Grigoriou O, Baka S, Makrakis E, Hassiakos D, et al. Prevalence of clinical vaginal candidiasis in a university hospital and possible risk factors. Eur J Obstet Gynecol Reprod Biol. 2006;126:121-125.
[Crossref] [Google Scholar] [PubMed]
- Ghaddar N, El Roz A, Ghssein G, Ibrahim JN. Emergence of vulvovaginal candidiasis among Lebanese pregnant women: prevalence, risk factors, and species distribution. Infect Dis Obstet Gynecol. 2019.
[Crossref] [Google Scholar] [PubMed]
- Zheng Y, Yu Q, Lin Y, Zhou Y, et al. Global burden and trends of sexually transmitted infections from 1990 to 2019: an observational trend study. Lancet Infect Dis. 2022;22:541-551.
[Crossref] [Google Scholar] [PubMed]
- Al-Naggar RA, Al-Jashamy K. Perception of undergraduate university students towards sexually transmitted diseases: A qualitative study. J Mens Health. 2011;8:S87-S90.
- Laga M, Alary M, Behets F, Goeman J, et al. Condom promotion, sexually transmitted diseases treatment, and declining incidence of HIV-1 infection in female Zairian sex workers. Lancet. 1994;344:246-248.
[Crossref] [Google Scholar] [PubMed]




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.