Quantitative Evaluation of Macrophage Expression Using CD68 in Oral Submucous Fibrosis: An Immunohistochemical Study
- *Corresponding Author:
- Dr. Treville Pereira
Department of Oral and Maxillofacial Pathology and Microbiology, Dr. D. Y. Patil Dental College and Hospital, Sector 7, Nerul, Navi Mumbai - 400 706, Maharashtra, India.
E-mail: trevillepereira@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Background: Macrophages are important cells for the innate immunity. Circulating monocytes are attracted to tissues by chemotactic factors and become macrophages under the influence of their microenvironment. Several studies suggested that local and systemic upregulation of fibrogenic cytokines and downregulation of antifibrotic cytokine are central to the pathogenesis of oral submucous fibrosis (OSMF). Currently, there have been no attempts made to elucidate the presence and role of macrophages in OSMF. Aim: Our aim was to study the expression of CD68 in OSMF patients and to investigate the possible correlation of macrophages using CD68 in various histopathological grades of OSMF. Subjects and Methods: A prospective case–control study which included 40 patients was conducted after obtaining informed consent and Ethical Committee clearance. Ten cases were normal control and thirty cases had OSMF. Biopsy was performed and a quantitative study of macrophages was done using CD68 antigen and was immunohistochemically localized. Statistical analysis was carried out using the Statistical Package for Social Sciences (SPSS) 17.0 version (SPSS Inc., Chicago, IL, USA). Results: OSMF was observed in male patients of a younger age group. The macrophage number in the patients of intermediate and advanced stage of OSMF was higher than that of the controls which was statistically significant (P < 0.05). Conclusion: The findings of this study suggest that CD68 plays a vital role in the pathogenesis of OSMF and can be regarded as a useful marker for assessing the progress of the disease.
Keywords
CD68, Macrophage, Oral, Submucous fibrosis
Introduction
Oral squamous cell carcinoma (OSCC) accounts for about 90% of all oral neoplasms. Despite advanced therapeutic approaches, the 5 years survival rate of patients with OSCC remains <50%.[1] Cancer research has provided us with a wide range of biological and chemical agents that play a vital role in the molecular pathogenesis of OSCC. However, we lack a reliable molecular marker that predicts both early diagnosis and prognosis of this devastating disease. Oral cancers may be preceded by clinically evident premalignant lesions or conditions particularly erythroplakia, leukoplakias, actinic cheilitis, oral submucous fibrosis (OSMF), and lichen planus.[2] Submucous fibrosis is an insidious, chronic disease affecting any part of the oral cavity, and sometimes the pharynx. OSMF is a high-risk precancerous condition characterized by changes in the connective tissue fibers of the lamina propria and deeper parts leading to the stiffness of the mucosa and restricted mouth opening. OSMF has been reported almost exclusively among Indians living in India and among other Asians, with a reported prevalence ranging up to 0.4% in Indian rural population.[3,4]
It is apparent that fibrosis and hyalinization of subepithelial tissues account for most of the clinical features encountered in this condition. Moreover, a substantial amount of research on elucidating the etiology and pathogenesis appears to have focused on changes in the extracellular matrix.[5] It was logical to hypothesize that the increased collagen synthesis or reduced collagen degradation as possible mechanisms in the development of the disease. The continuous contact of the alkaloids in the quid on the oral mucosa causes chronic inflammation leading to activation of macrophages and T cells and an increase in the level of cytokines such as interleukine 6 (IL-6), tumor necrosis factor (TNF), interferon α (IFN-α), and transforming growth factor beta. Further, microtrauma produced by the friction of coarse fibers of areca nut also facilitates diffusion of the alkaloids into the subepithelial connective tissue resulting in juxta epithelial inflammatory cell infiltration.[6]
Fibrogenic cytokines are secreted by activated macrophages or T lymphocytes are very important in the development of fibrotic disorders.[7] Circulating monocytes are attracted to tissues by chemotactic factors and become macrophages under the influence of their microenvironment.[8] Several studies suggest that local and systemic upregulation of fibrogenic cytokines and downregulation of antifibrotic cytokine are central to the pathogenesis of OSMF.[7]
Currently, there have been no attempts made to elucidate the presence and role of macrophages in OSMF. The present study aims to assess the severity of the disease by the clinical staging and to correlate clinical staging with histopathological staging. Further this study aims to evaluate the presence of macrophages in various histological stages of OSMF using CD68 antigen, which has been suggested as the most reliable marker for macrophage differentiation.
Subjects and Methods
This prospective case–control study was carried out in the Oral and Maxillofacial Department of Dr. D. Y. Patil Dental College, Nerul, Navi Mumbai, India. The study was carried out on 40 subjects, which were divided into two groups. The study group comprised of 30 subjects with OSMF. Subjects had a history of oral tissue abuse habits such as tobacco chewing, smoking, areca nut chewing, and alcohol consumption. Relevant history of each subject was recorded thoroughly to rule out any systemic illness or any prior therapy. Subjects who had taken antibiotics in the course of the previous month for any tooth infection, those on anticoagulant therapy, chemotherapy, radiation therapy were excluded from the study. The study was carried out over a period of 6 months. After thorough evaluation, 10 subjects for the control group were selected from age and sex matched subjects. These subjects were ruled out for the presence of any systemic illness by taking a thorough history. Further, these subjects did not have any oral tissue abuse habits or any obvious oral lesions. Written informed consent and Institutional Ethical Committee clearance was obtained for the study.
A clinical confirmation of diagnosis was performed based on the presence of one or more of the following parameters:
• Palpable fibrous bands
• Leathery feeling of the mucosae: Texture of the mucosae was determined by palpation using the index finger
• Mobility of the tongue, assessed according to the ability to protrude
• Burning sensation on eating spicy food: Only the presence or absence was determined and no attempt was made to quantify.
Examination of fibrous bands by observation, palpation, and resistance to stretching was carried out. Clinical grading of severity of fibrosis was carried out based on the study conducted by Lai et al., in which the OSMF population was divided based on the interincisal distance as:
• Group A - >35 mm
• Group B - between 30 and 35 mm
• Group C - between 20 and 30 mm
• Group D - <20 mm.[9]
After selection of suitable patients, site for biopsy was selected and incisional biopsy was performed under aseptic conditions and local anesthesia. Biopsy specimens were preserved in 10% neutral buffered formalin solution. The biopsied tissue of the study groups were processed and embedded in paraffin wax. The paraffin blocks were sectioned with a semi-automatic microtome (Leica, Germany) into two tissue sections of 3 μm thickness. One section was stained with hematoxylin and eosin (H and E) for comparative purpose using the following procedure.
Staining procedure for H and E:
• The sections were deparaffinized in xylene-Ι and xylene-ΙΙ for 10 min each and were then dehydrated in various grades of alcohol for 5 min each
• After water wash for 10 min, the slides were stained with Harris’s hematoxylin stain for 7 min
• Again, water washed for 10 min and after differentiation in acid alcohol, the slides were dipped in lithium carbonate for bluing for 5 min and were stained with eosin for 15 s
• Dehydrated with graded alcohol, cleared in xylene and mounted.
Further, the H and E stained sections were histopathological graded using the criteria given by Utsunomiya et al.,[10] which is based on the concept of the Pindborg and Sirsat grading system as follows.
• Early stage 1: Large number of lymphocytes in subepithelial connective tissue zone along with myxedematous changes
• Intermediate stage 2: Granulation changes close to the muscle layer and hyalinization appears in the subepithelial zone where blood vessels are compressed by fibrous bundles; reduced inflammatory cells in subepithelial layer
• Advanced stage 3: Inflammatory cell infiltrate hardly seen. Number of blood vessels dramatically small in the subepithelial zone. Marked fibrous areas with hyaline changes extending from subepithelial to superficial muscle layers. Atrophic degenerative changes start in muscle fibers.
Immunohistochemistry
The other 3 μm section of the tissue were cut and transferred to APES (Sigma-Aldrich Chemical Co., USA) coated slides and incubated overnight at room temperature. After warming in a slide warmer for 15 min, the sections were deparaffinized in three changes of fresh xylene each for 5 min followed by dehydration in a series of absolute alcohol each for 5 min. Endogenous peroxidases were blocked with peroxide block (BioGenex Life sciences Pvt., Ltd, CA, USA) for 15 min at room temperature and washed in distilled water followed by citrate buffer (pH 6.0) wash for 10 min. Antigen retrieval was undertaken with a help of BioGenex antigen retrieval system. The sections were immersed in citrate buffer solution and placed into the BioGenex antigen retrieval system and heated for 15 min. The system was allowed to cool to room temperature by placing it under running tap water and later the slides were washed with distilled water for 5 min. With an intention to block endogenous biotin, the sections were incubated with a blocking agent (BioGenex Life sciences Pvt., Ltd., CA, USA) for 15 min. Excess power block solution was drained and the sections were incubated with primary monoclonal antibody of CD68 (BioGenex life sciences Pvt., Ltd, CA, USA) for 1 h and later thoroughly washed with citrate buffer. For further enhancement of the staining, the sections were then incubated with the anti-mouse secondary antibody (super enhancer) for 30 min followed by two consecutive buffer washes; each for 5 min. Horseradish peroxidase (BioGenex Life Sciences Pvt., Ltd., CA, USA) was added to the sections and incubated for 30 min. The chromogen diaminobenzidine was prepared just prior to use by mixing one drop of chromogen to 1 ml of the buffer in a mixing vial and later added over the sections. After 5 min, the sections were washed in buffer followed by water and counterstained with Harris hematoxylin, air dried, cleared, and mounted with dibutyl phthalate xylene. Langerhans cell histiocytosis tissue was used as positive control.
Interpretation of staining
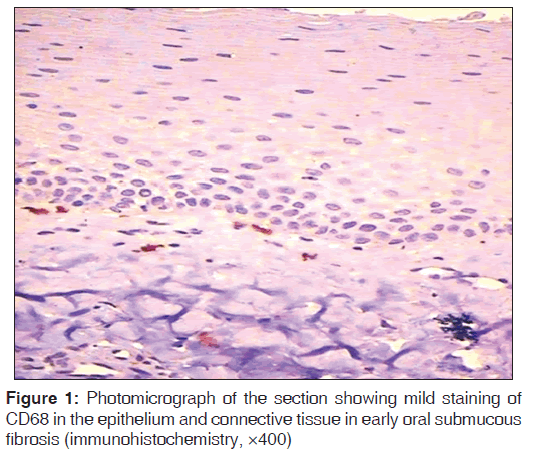
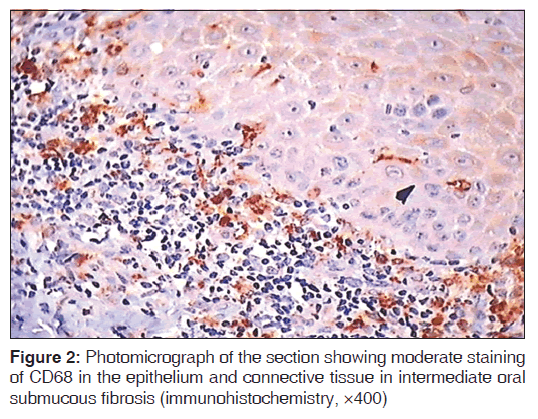
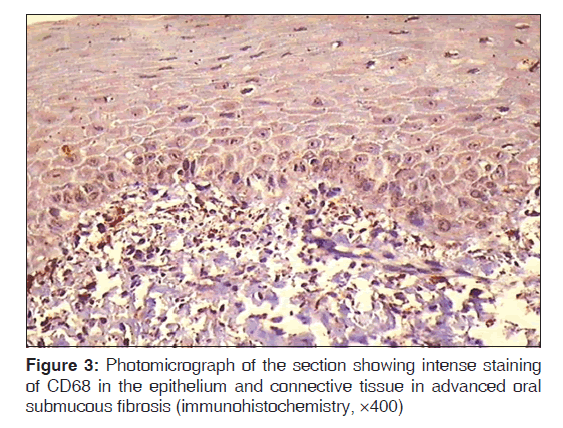
Epithelial and subepithelial cells positive for CD68 were regarded as macrophages because CD68, a glycoprotein found in lysosomes and to a lesser extent on surface membranes, is highly expressed by macrophages exhibiting brown cytoplasmic or membranous staining which were counted as positive for the expression of CD68. Macrophages were counted using a Leica research microscope with provision for photomicrograph (Model no. DM 1000 LED, Germany). The stained sections were first screened at low power (×10) to determine the areas of most intense staining for CD68. Macrophage cell counting was then performed under ×40 magnification. Ten high-power fields (HPFs) with highest number of macrophages were chosen. The representative areas were carefully scanned from left to right of every slide to avoid recounting of same areas. The macrophage for each case was the average of the number of macrophages in these 10 chosen HPFs and expressed as the number of macrophages per HPF (macrophages/HPF) [Figures 1-3]. The mean of 10 values was calculated and expressed as mean (standard deviation). All immunohistochemistry (IHC)-stained slides along with the corresponding H and E slides were evaluated by two qualified observers to minimize the subjective bias.
Statistical methods
Statistical analysis of the data was carried out using the Statistical Package Social Sciences (SPSS) 17.0 software (SPSS Inc., Chicago, IL, USA). Discrete statistical data was analysed by Pearson’s Chi-square test, student ‘t’ test, standard deviation and the mean value. Independent sample ‘t’ test was used to test the difference between the mean macrophage cell density in study group with macrophage density of control group in the epithelium and connective tissue. P < 0.05 was considered as statistically significant.
Observations and Results
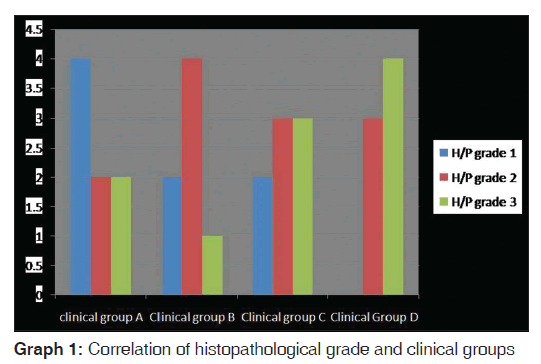
The study comprised of 30 subjects with OSMF and 10 subjects with no oral habits. The study population comprised of 24 males and 6 females with a mean age of 29 years and the control group comprised of 6 males and 4 females. In the present study, 10 subjects were in 19–25 years age range, 8 were in 26–31 years age range, 6 in 32–38 years age range, and 6 in age range of 39–45 years. The maximum numbers of OSMF subjects were found in the age group of 19–25 years. The study group as well as control group showed a male predominance. Twenty-six subjects in the study group had a mixed food habit while 4 subjects had only a vegetarian diet. Twenty subjects in the study group gave a history of eating spicy food on a regular basis, while 10 subjects ate spicy food occasionally. Most of the subjects in the study group had a combination of tobacco chewing habits. Six subjects chewed areca nut with gutkha, 11 had a habit of chewing areca nut with tobacco, and 7 had a habit of chewing only areca nut. Twenty-eight subjects in the study group had palpable bands on the buccal mucosa followed by 21 in the retromolar area, 14 in the circumoral region, 17 on the soft palate, and 1 in the floor of the mouth. Clinical grades revealed that out of 30 study group patients, 8 had Grade A OSMF, 7 had Grade B OSMF, 8 had Grade C OSMF, and 7 had Grade D OSMF. Histopathological grades in the study group revealed, 8 were in stage 1, 12 were in stage 2, and 10 were in stage 3. We observed that the majority of study group falls in stage 2. However, when a comparison of the clinical groups and the histopathological grades was done, it was statistically insignificant (P = 0.26) [Table 1 and Graph 1].
| Histopathological | Clinical grading | Total | P | |||
|---|---|---|---|---|---|---|
| grades | Group A | Group B | Group C | Group D | ||
| 1 | 4 | 2 | 2 | 0 | 8 | 0.26 not significant |
| 2 | 2 | 4 | 3 | 3 | 12 | |
| 3 | 2 | 1 | 3 | 4 | 10 | |
| Total | 8 | 7 | 8 | 7 | 30 | |
Table 1: Correlation of histopathological grades and clinical groups
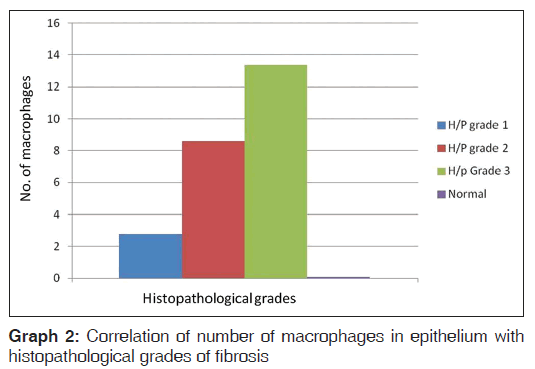
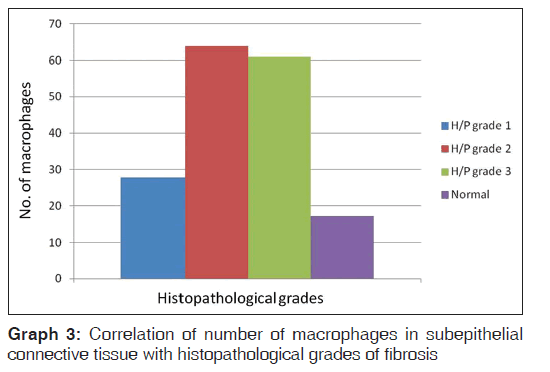
When a comparison of the number of macrophages in epithelium with the histopathological grades of fibrosis was done we noted that in the study group, histopathological stage1 had 2.75 (0.9), histopathological stage 2 had 8.6 (2.23), and histopathological stage 3 had 13.4 (2.9) number of macrophages, while in the control group the number of macrophages was 0.1 (0.2). The mean macrophage cell density in study group epithelium was significantly higher than the corresponding mean cell macrophage density in epithelium of control group with P = 0.04, which was statistically significant [Table 2 and Graph 2]. When a comparison of the number of macrophages in the subepithelial connective tissue with the histopathological grades of fibrosis was done, we noted that the histological stage 1 had 27.8 (5.17), histological stage 2 had 64 (4.09), while histological stage 3 had 60.9 (2.08) number of macrophages. The mean cell density of macrophages in subepithelial connective tissue in a study group was significantly higher than the corresponding mean cell density in control group with P = 0.04, which was statistically significant [Table 3 and Graph 3].
| Number ofmacrophages | Histopathological grades | P | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | Normal | ||
| Mean value | 2.75 (0.9) 8.6 (2.23) 13.4 (2.9) | 0.1 (0.2) | 0.04 significant | ||
Table 2: Correlation of number of macrophages in epithelium with histopathological grades of fibrosis
| Number ofmacrophages | Histopathological grades | P | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | Normal | ||
| Mean value | 27.8 (5.17) 64 (4.09) 60.9 (2.08) 17.3 (19.1) | 0.04 significan | |||
Table 3: Correlation of number of macrophages in subepithelial connective tissue with histopathological grades of fibrosis
Discussion
OSMF is a chronic disease of oral mucosa characterized by inflammation and progressive fibrosis of the lamina propria and deeper connective tissues, followed by stiffening of an otherwise yielding mucosa resulting in difficulty in opening the mouth.[11]
The pathogenesis of OSMF is believed to be multifactorial. The most important risk factor, however, is the chewing of betel quid and this had been supported by epidemiological, case–control, experimental animal, and tissue culture studies as well. A multifactorial model for the pathogenesis of OSMF such as iron and nutritional deficiencies, chronic candidiasis, tobacco, lime, betel quid, genetic abnormalities, herpes simplex virus, human papillomavirus, autoimmunity, etc., have been postulated and are known to have either direct effect in causing OSMF or an indirect effect by mediating the immune system which is compromised in OSMF.[6]
Macrophages too have also been implicated in the pathogenesis of OSMF.[7] Haque et al. demonstrated that the peripheral blood mononuclear cells from OSMF patients secrete increased levels of stimulated IL-1β, TNF-α, IL-6, IL-8, and a decreased level of stimulated IFN-g. The immune competent cells, especially the macrophages and lymphocytes, are likely the main source of cytokine synthesis. However, review of the literature showed very few studies carried out in this aspect. The main goal of the present study were to assess whether there was a significantly higher number of CD68 macrophage cells in OSMF tissues and to investigate the relationship of macrophage densities in the subepithelial connective tissue and in the epithelium ofOSMF specimens in relation to various oral habits practiced by the OSMF subjects.
In a study by Ranganathan et al., the maximum number of patients were in the age group of 26–35 with a mean age of 32.4 years.[12] Ariyawardana et al.[13] found a maximum distribution in 25–34 years, while Kiran et al.[14] found a similar distribution, whereas Pindborg[15] reported the maximum number of OSMF cases in the age group of 40–49 years. The present study correlated with few of the studies carried out by other authors.
Kiran et al.[14] in their study showed a gender distribution of 6:1, while 4.9:1 was observed by Hazarey et al.[16] and 4.6:1 was seen by Ariyawardana et al.[13] Sinor et al.[17] observed a ratio of 29:1, and Ranganathan et al.[12] observed a ratio of 9.9:1. However, most studies showed a male predominance. Pindborg[15] reported a female predominance (female: male = 8:3). The gender distribution in the present study was 4:1 (male: female). The sex incidence ratio varies from study to study due to varying habits in various populations. Females in the villages tend to use mainly local pan and males tend to use processed betel nut along with the local pan. In the present study, female patients were seen less probably because very few females have the habit of chewing betel nut in and around Navi Mumbai.
Based on the clinical staging most of the study group were in the Grade A and Grade C. Haider et al.[18] and Hazarey et al.[16] also found that almost half of the patients in their study were in clinical stage C. However, their staging criteria were different as compared to the present study.
Haque et al.[19] characterized the inflammatory cell infiltrates in the lesional oral mucosa from OSMF patients by IHC. They found that activated T lymphocytes, especially activated helper/inducer T lymphocytes, are the major cell population; macrophages are the minor cell population. In the present quantitative study, we further found a significant increase in macrophage densities in the subepithelial connective tissue of OSMF specimens compared to those in control specimens.
The significant increase in the number of macrophages in the subepithelial connective tissue of OSMF specimens suggested that the cellular immune response play an important role in the pathogenesis of OSMF.
Moreover, IL-1β and TNF-α have been demonstrated to upregulate mRNA expression of collagen types I and III. Intradermal injections of TNF-α stimulate the accumulation of fibroblasts and collagen. TNF-α has also been shown to inhibit adherence and phagocytosis of collagen. Similarly, both IL-6 and IL-8 have also been implicated in the development of fibrosis. On the contrary, IFN-g is an antifibrotic cytokine that can inhibit collagen synthesis. Improvement of keloids and hypertrophic scars by the intralesional IFN-g treatment has been reported. Furthermore, local injections of IFN-g reduce the contracture formation and facilitate the mouth opening in the OSMF patients.[7] Haque et al. investigated the presence and distribution of inflammatory cells and MHC class II antigen expression by epithelial and immunocompetent cells using a three-stage immunoperoxidase method on thirty frozen sections of OSMF. All tissues were investigated using antibodies to T cells (CD3), T helper/inducer cells (CD4), T suppressor/ cytotoxic cells (CD8), B cells (CD20), naive T cells and monocytes (CD45RA), macrophages, Langerhans’ cells (CD68), and human leukocyte antigen Human leukocyte antigen-DR (HLA-DR)-positive cells (HLA-DR alpha). The predominant cell populations detected in normal tissues were CD3, CD4, and HLA-DR-positive cells. The cell population detected in OSF showed higher numbers of CD3 and HLA-DR-positive cells compared with those of the normal tissues. The pattern of staining for CD4-positive cells in OSF tissues was similar to that of CD3-positive cells both in the epithelium and connective tissue and was higher than that in normal tissues. A few scattered CD8-positive cells and only occasional CD20- and CD68-positive cells were seen in OSMF sections. The presence of these immunocompetent cells and high ratio of CD4 to CD8 in OSMF tissues suggest an ongoing cellular immune response leading to a possible imbalance of immunoregulation and alteration in local tissue architecture.[20] Bôas et al. compared the microvascular density (MVD) and infiltrating macrophage density (IMD) in oral OSCCs with different histological grades using antibodies such as von Willebrand factor and CD68. However, a significant correlation between MVD and IMD could not be obtained suggesting that angiogenesis does not depend on the number of macrophages present in OSCC, but their predominant phenotype.[21] El-Rouby studied the association between tumor-associated macrophages (TAMs) and angiogenesis in formalin-fixed, paraffin-embedded archival material of OSCC, and oral verrucous carcinoma. TAMs shown by IHC for CD68 and microvessels demonstrated by IHC for CD31 were quantified using an image analyzer computer system. He observed an increased TAMs density associated with angiogenesis in higher histopathological grades in oral cancer.[22] In the present study, manual counting of the macrophages was done. Wehrhan et al. analyzed the macrophage polarization in cervical lymph nodes of OSCC patients. The study revealed an influence of OSCC on the macrophage polarization in regional lymph nodes. Markers of malignant behavior in the primary tumor were associated with a shift of macrophage polarization in lymph nodes from the anti-tumoral M1 type to the tumor-promoting M2 type.[23] Pettersen et al. studied the activation state of TAMs in cutaneous SCC where CD163 was identified as a more abundant, sensitive, and accurate marker of TAMs when compared with CD68.[24] In the present study, only CD68 was used however we found a similar correlation with different histological grades of OSMF.
Conclusion
Macrophages are important cells in wound healing, providing aid for tissue growth and angiogenesis. The TAM derived from the peripheral blood monocytes exhibit tumoricidal activity toward one fraction of the cancer cells. Clinically, the TAM level has been correlated with poor prognosis in several human cancers. The cellular immune response with elevated production of fibrogenic cytokines and reduced production of antifibrotic cytokines play an important role in the pathogenesis of OSMF. The cellular immune response by macrophages do play a role in pathogenesis of OSMF, but the role of macrophages in the repair process and the resolution of inflammation is still very poorly understood, and for this, the development of better experimental models are required.
In the present study, the macrophage expression showed a progressive increase in number and had a positive correlation with histologic grading of OSMF. The expression of macrophage can thus be used to evaluate cases of oral malignancy though neither as a strong arbiter nor as a distinct diagnostic test. Targeting macrophages in the context of oral disease is therefore a prospective area for future research and development in cell-based therapeutic interventions that are aimed at redirecting macrophage effect or functions which could benefit patients with inflammatory disease and cancer. Thus, the present study forms a nidus for further research to be conducted to affirm the absolute utility of macrophage estimation in OSMF using CD68 as an immunohistochemical marker. Above all, studies including the follow-up of patients are necessary; in order to understand the true value of the macrophage density as a prognostic parameter in OSMF.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Markopoulos AK. Current aspects on oral squamous cell carcinoma. Open Dent J 2012;6:126-30.
- Scully C, Bagan JV, Hopper C, Epstein JB. Oral cancer: Current and future diagnostic techniques. Am J Dent 2008;21:199-209.
- Shivakumar GC, Sahana S. Correlation between the functional and histological staging of oral submucous fibrosis. J Indian Acad Oral Med Radiol 2010;22:133-5.
- Rajendran R, Raju GK, Nair SM, Balasubramanian G. Prevalence of oral submucous fibrosis in the high natural radiation belt of Kerala, south India. Bull World Health Organ 1992;70:783-9.
- Chiu CJ, Chang ML, Chiang CP, Hahn LJ, Hsieh LL, Chen CJ. Interaction of collagen-related genes and susceptibility to betel quid-induced oral submucous fibrosis. Cancer Epidemiol Biomarkers Prev 2002;11:646-53.
- Sudarshan R, Annigeri RG, Sree Vijayabala G. Pathogenesis of oral submucous fibrosis: The past and current concepts. Int J Oral Maxillofac Pathol 2012;3:27-36.
- Chiang CP, Wu HY, Liu BY, Wang JT, Kuo MY. Quantitative analysis of immunocompetent cells in oral submucous fibrosis in Taiwan. Oral Oncol 2002;38:56-63.
- Lu CF, Huang CS, Tjiu JW, Chiang CP. Infiltrating macrophage count: A significant predictor for the progression and prognosis of oral squamous cell carcinomas in Taiwan. Head Neck 2010;32:18-25.
- Lai DR, Chen HR, Lin LM, Huang YL, Tsai CC. Clinical evaluation of different treatment methods for oral submucous fibrosis. A 10-year experience with 150 cases. J Oral Pathol Med 1995;24:402-6.
- Utsunomiya H, Tilakaratne WM, Oshiro K, Maruyama S, Suzuki M, Ida-Yonemochi H, et al. Extracellular matrix remodeling in oral submucous fibrosis: Its stage-specific modes revealed by immunohistochemistry and in situ hybridization. J Oral Pathol Med 2005;34:498-507.
- Ahmad MS, Ali SA, Ali AS, Chaubey KK. Epidemiological and etiological study of oral submucous fibrosis among gutkha chewers of Patna, Bihar, India. J Indian Soc Pedod Prev Dent 2006;24:84-9.
- Ranganathan K, Devi MU, Joshua E, Kirankumar K, Saraswathi TR. Oral submucous fibrosis: A case-control study in Chennai, South India. J Oral Pathol Med 2004;33:274-7.
- Ariyawardana A, Athukorala AD, Arulanandam A. Effect of betel chewing, tobacco smoking and alcohol consumption on oral submucous fibrosis: A case-control study in Sri Lanka. J Oral Pathol Med 2006;35:197-201.
- Kiran Kumar K, Saraswathi TR, Ranganathan K, Uma Devi M, Elizabeth J. Oral submucous fibrosis: A clinico-histopathological study in Chennai. Indian J Dent Res 2007;18:106-11.
- Pindborg JJ. Oral submucous fibrosis: A review. Ann Acad Med Singapore 1989;18:603-7.
- Hazarey VK, Erlewad DM, Mundhe KA, Ughade SN. Oral submucous fibrosis: Study of 1000 cases from central India. J Oral Pathol Med 2007;36:12-7.
- Sinor PN, Gupta PC, Murti PR, Bhonsle RB, Daftary DK, Mehta FS, et al. A case-control study of oral submucous fibrosis with special reference to the etiologic role of areca nut. J Oral Pathol Med 1990;19:94-8.
- Haider SM, Merchant AT, Fikree FF, Rahbar MH. Clinical and functional staging of oral submucous fibrosis. Br J Oral Maxillofac Surg 2000;38:12-5.
- Haque MF, Harris M, Meghji S, Barrett AW. Immunolocalization of cytokines and growth factors in oral submucous fibrosis. Cytokine 1998;10:713-9.
- Haque MF, Harris M, Meghji S, Speight PM. An immunohistochemical study of oral submucous fibrosis. J Oral Pathol Med 1997;26:75-82.
- Bôas DS, Takiya CM, Gurgel CA, Cabral MG, Santos JN. Tumor-infiltrating macrophage and microvessel density in oral squamous cell carcinoma. Braz Dent J 2013;24:194-9.
- El-Rouby DH. Association of macrophages with angiogenesis in oral verrucous and squamous cell carcinomas. J Oral Pathol Med 2010;39:559-64.
- Wehrhan F, Büttner-Herold M, Hyckel P, Moebius P, Preidl R, Distel L, et al. Increased malignancy of oral squamous cell carcinomas (oscc) is associated with macrophage polarization in regional lymph nodes – An immunohistochemical study. BMC Cancer 2014;14:522.
- Pettersen JS, Fuentes-Duculan J, Suárez-Fariñas M, Pierson KC, Pitts-Kiefer A, Fan L, et al. Tumor-associated macrophages in the cutaneous SCC microenvironment are heterogeneously activated. J Invest Dermatol 2011;131:1322-30.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.