Revisiting Plummer Vinson Syndrome
- *Corresponding Author:
- Dr. Dilip Gude, Registrar
Internal Medicine, AMC, 3rd Floor, Medwin Hospital, Chirag Ali lane, Nampally, Hyderabad - 500001, Andhra Pradesh, India.
E-mail: letsgo.dilip@gmail.com
Abstract
Plummer Vinson syndrome is a rare association of postcricoid dysphagia, upper esophageal webs, and iron deficiency anemia. Iron deficiency state has been hypothesized to play an etiological role. While literature review elucidates the resolution of dysphagia in most cases with iron therapy, we discuss our case where the dysphagia was resistant to such therapy and necessitated a mechanical dilatation.
https://marsbahislinki.com https://marsbetgiris.com https://mobilebahiss.com https://1xbetsgirisi.com https://onwinegiris.com https://betistegiris.com https://piacasinogiris.com https://holiganbahiss.com https://holiganbetting.com https://girisholigan.com https://mostbetegiris.com https://mariobetoyna.com https://meritgirisi.com https://meritkinge.com https://kingmerite.com https://nanogiris.com https://casinoplusa.com https://casinoplusgiris.com https://betriyal.net https://pluscasinogiris.com
Keywords
Esophageal webs, Iron deficiency anemia, Plummer Vinson syndrome
Introduction
Plummer Vinson syndrome (also called Paterson-Brown-Kelly syndrome (PVS)) is a rare entity comprising upper esophageal webs and iron deficiency anemia. First described in 1912, PVS affects mostly women of Caucasian origin (age group of fourth to seventh decade).[1] Most researchers believe that iron therapy reverses the dysphagia.[2] But there are some conflicting reports on cervical webs and either overt or latent iron deficiency suggesting no correlation between the same.[3] We present our case of PVS in which the dysphagia did not respond to iron replenishment and ultimately required an endoscopic dilatation. We explore the association of iron replenishment and reversibility of dysphagia.
Case Report
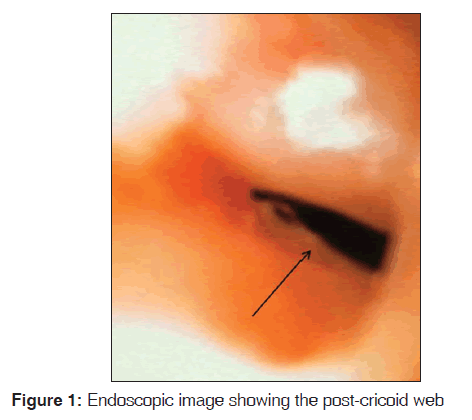
A 49-year-old female presented with difficulty in swallowing and easy fatigability from 3 months. The dysphagia initially was mainly to solids. There was no history of malaena or hematochezia. On examination her pulse rate was regular, 98/min, and blood pressure was 110/80 mm of Hg. She had pallor and koilonychia. Systemic examination was unremarkable. Lab reports showed hemoglobin (Hb)- 8.8 g/dl, total leukocyte count (TLC)-6400/μL, platelet count-140,000/μL. Mean corpuscular volume (MCV)-60.7 fl, mean corpuscular hemoglobin (MCH)-19.2 pg/cell, mean corpuscular hemoglobin concentration (MCHC)-31.8 g/dl; serum creatinine-0.8 mg/dl, total bilirubin-0.8 mg/dl, (direct-0.11 mg/dl), SGPT-8 U/L. Fecal occult blood test was negative and stool for parasites was negative as well. Serum iron was 40 μg/dl; Total iron binding capacity was 170 μg/dl; transferrin saturation was 10% and serum ferritin was 15 ng/ml. Peripheral smear showed microcytic hypochromic picture. Ultrasonography of abdomen and pelvis was normal. Barium swallow was suggestive of a web at C5 level at anterior aspect of pharyngo-esophageal junction. Upper GI endoscopy showed a smooth, circular, whitish narrowing suggestive of a post cricoid web at the C5 vertebra-level [Figure 1]. There was no evidence suggestive of other causes of iron deficiency anemia such as malabsorption, malnutrition, use of NSAIDs and abnormal vaginal bleeding. Absence of iron overload, no hemolysis, no history suggestive of lead exposure, use of isoniazid or any chronic diseases make the alternative diagnoses such as sideroblastic anemias, thalassemias, warm antibody autoimmune hemolytic anemia, anemia of chronic disease, lead poisoning, and pyridoxine deficiency less likely. A diagnosis of PVS was made. Upon patient’s insistence, endoscopic dilatation was deferred and the patient was discharged on oral iron supplements (ferrous ascorbate 100 mg). Patient was lost to follow up until 6 months when patient presented again with complaints of non-resolving dysphagia and that the dysphagia progressed to liquids. Work up this time showed that Hb improved to 10.8 g/dl, MCV-80.2 fl, MCH-28 pg/cell, and MCHC-33.4 g/dl. Fluoroscopy-guided dilatation of the hypopharyngeal web was done (no evidence of mass/ulceration or thickening found on fluoroscopy). Dysphagia subsided and patient’s general condition improved. She was able to swallow solids and liquids at the time of discharge. Oral iron supplements were continued and she was counseled for frequent follow-ups.
Discussion
PVS is a manifestation of severe, long-term, iron deficiency anemia causing dysphagia because of esophageal webs. While esophageal webs are thin mucosal folds (covered with squamous epithelium) protruding into the lumen of proximal esophagus, esophageal rings occur more distally and are further classified as the muscular A rings (upper part of distal esophagus), mucosal Schatzki B rings, and the distal-most C rings.[3] Dysphagia from the webs of PVS is commonly painless and intermittent or progressive (as described in our patient). The characteristics may also include glossitis, glossopyrosis, glossodynia, angular cheilitis, koilonychia, fragility, thinning of nails, and brittle hair. Rare manifestations such as clubbing instead of koilonychia and tortuous esophagus in addition esophageal webs have also been described.[4] Symptoms secondary to anemia such as pallor, fatigue, and weakness may also dominate the clinical picture.
Although autoimmune, genetic, infectious, and nutritional factors have been proposed,[1] iron deficiency garnered importance as the major etiologic factor as most cases have shown improvement of the dysphagia after iron therapy. The high cellular turnover rate in the epithelium of the upper digestive tract makes it vulnerable to iron deficiency because of the deficiency of the iron dependent enzymes.[5] This depletion of the oxidative enzymes of epithelial cells, free radical stress, and DNA damage may be responsible for the epithelial changes (above described) observed in patients with PVS. This also predisposes the patients to malignant change especially squamous cell carcinomas.[5] Iron deficiency is believed to decrease the contraction amplitude of the esophageal muscle resulting in motility impairment. Some degree of pyridoxine deficiency is also believed to contribute to PVS.[6] Slower transit times have been recorded at the proximal and middle parts of the esophagus of PVS patients compared to healthy volunteers.[7]
The above discussed hypothesis that iron deficiency anemia contributing to esophageal webs may also apply in our case as an etiologic factor initially. But our case being chronic long-standing anemia, the inflammatory pathology and its fibrotic sequelae have been expectantly refractory to iron replacement although the blood indices have normalized. Very few cases have been reported that were recalcitrant to iron replenishment. Dysphagia in such settings necessitates mechanical dilatation in adjunction with iron therapy. In most cases, one session of such dilatation is usually enough for long-term relief but, rarely, multiple sessions may also be warranted.[1]
The approach begins with lab evaluation of CBC count, iron and ferritin studies, antigliadin and antiendomysial antibodies (to rule out celiac sprue). Barium-swallow studies and fluoroscopic evaluation suggest the diagnosis and the degree of stenosis. Esophago-gastro-duodenoscopy helps obtain histological samples to rule out other disorders, confirms the diagnosis and also helps therapeutically in the dilation of webs. Mercury/tungsten filled bougies (Maloney/Hurst), bougienage dilators (bougie passed over guidewire; Savary-Gilliard or American) and through-the-scope (balloon dilators) are the esophageal dilators commonly available.[8] Clinical spectrum of complications of esophageal dilation may range from odynophagia and hematemesis to severe sepsis and fatal consequences secondary to perforation, bleeding, aspiration and mediastinitis.
A variety of disorders have been known to associate PVS such as celiac disease, inflammatory bowel disease, pernicious anemia, thyroid disease, Sjogren’s disease, and rheumatoid arthritis.[7,9,10]
Iron replacement is recommended at least until normalization of the hematocrit and ferritin levels. Apart from endoscopic dilatation, argon plasma coagulation therapy of esophageal webs has been tried with reasonable success.[11] The prognosis is mostly good at short-term follow ups. The benefit from dilatation may be short-lived and “satisfactory resolution” of dysphagia can be concluded only up on longer follow-up periods.
Conclusion
From our experience we would like to highlight that iron replacement does not necessarily reverse the dysphagia in all the cases of PVS and that close monitoring of the web is mandated to watch for malignant change.
Acknowledgement
We thank our colleagues and staff of Internal Medicine and Critical Care, Medwin Hospitals.
Source of Support
Medwin Hospital, Nampally, Hyderabad, AP, India.
Conflict of Interest
None declared.
References
- Novacek G. Plummer-Vinson syndrome. Orphanet J Rare Dis 2006;1:36.
- Chisholm M. The association between webs, iron and post-cricoid carcinoma. Postgrad Med J 1974;50:215-9.
- Zervos X, Pyrsopoulos NT, Qureshi WA, Talavera F, Bank S, Mechaber AJ, et al. Esophageal webs and rings, eMedicine, 2011. Available from: http://emedicine.medscape.com/ article/186561-overview#showall. [Last retrieved on 2012 Jun 22].
- Makharia GK, Nandi B, Garg PK, Tandon RK. Plummer Vinson syndrome: Unusual features. Indian J Gastroenterol 2002;21:74-5.
- Donohue-Cornejo A, Guzmán-Gastelum DA, Constandse-Cortéz D, Gaitán-Cepeda LA, Escalera CR. Squamous cell carcinoma in the tongue and Plummer-Vinson syndrome. A case report. Revista Odontológica Mexicana 2011;15:189-92.
- Jacobs A, Cavill IA. Pyridoxine and riboflavin status in the Paterson-Kelly syndrome. Br J Haematol 1968;14:153-60.
- Dinler G, Tander B, Kalayci AG, Rizalar R. Plummer-vinson syndrome in a 15-year-old boy. Turk J Pediatr 2009;51:384-6.
- Egan JV, Baron TH, Adler DG, Davila R, Faigel DO, Gan SL, et al.; Standards of practice committee. Esophageal dilation. Gastrointest Endosc 2006;63:755-60.
- Ouakaa-Kchaou A, Jebali S, Elloumi H, Gargouri D, Kochlef A, Romani M, et al. Association of Sjögren’s syndrome and plummer vinson syndrome. Rev Med Interne 2011;32:e21-2.
- Ogunbiyi OA, El Tahir MI. Paterson-Brown Kelly syndrome. Ann Saudi Med 1996;16:130-4.
- Crespo Pérez L, Graus Morales J, Blesa Radigales C, Cano Ruiz A. Argon plasma coagulation therapy of upper esophageal web in a patient with plummer-vinson syndrome: A new therapeutical option. Med Clin (Barc) 2010;135:141-2.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.