Serum Leptin Level in Women with Polycystic Ovary Syndrome: Correlation with Adiposity, Insulin, and Circulating Testosterone
- *Corresponding Author:
- Dr. Jana Chakrabarti
Department of Biotechnology, Presidency University (Erstwhile Presidency College), 86/1, College Street, Kolkata, West Bengal, India
E-mail: jana_chakrabarti@ yahoo.co.uk
Abstract
Background: Leptin, an adipocyte‑derived hormone encoded by ‘ob’ gene, serves as a link relaying metabolic signals to the neuronal networks in the brain to modulate hypothalamo‑pituitary‑ovarian axis. Circulating leptin correlates strongly with obesity, which is frequently associated with polycystic ovarian syndrome (PCOS), a major form of dysovulatory infertility in women, characterized by endocrine abnormalities such as hyperandrogenism and inappropriate LH secretion. PCOS is also often associated with insulin resistance and hyperinsulinemia, features that are linked to leptin and its receptors. However, the relationship between leptin and gonadotropins, androgens, and insulin in PCOS is still controversial. Aim: Present investigation attempts to evaluate the interrelationship between serum leptin level with body mass index, insulin and with circulating testosterone in PCOS women. Subjects and Methods: Women diagnosed with either tubal block/male factor infertility (Control; n = 18) and with PCOS (n = 16), enrolled for in vitro fertilization and embryo transfer (IVF‑ET), were recruited for this study. All were reviewed for body mass index (BMI), endocrine milieu (including pituitary gonadotropins, TSH, prolactin, gonadal steroids, and insulin) and for circulating serum leptin. Interpretation of data was done using PRISM Statistical Software Package (PRISM Version 4.03@1992‑2005; GraphPad Software Inc). Results: Positive correlation was observed between serum leptin, BMI, and insulin in both the groups. Mean BMI, LH, and LH: FSH ratios were found elevated in the PCOS population. PCOS women also had significantly elevated androgens and fasting levels of insulin. Conclusion: Hyperleptinemia in PCOS women appears to be due to the positive correlation between serum leptin, BMI, and insulin.
Keywords
Body mass index, Hyperandrogenemia, Insulin resistance, Leptin, Polycystic ovary syndrome
Introduction
A critical body mass of adipose tissue is essential for the normal development of female reproductive functions.[1] Reproductive anomalies in young pre-menopausal women seem to occur as a consequence of the linear decline in adiposity. Obesity, on the other hand, has been shown to produce derangement of female reproductive functions and infertility.[2] But, the mechanistic link between body mass and reproductive functions is not yet clearly elucidated.[3] Leptin, an adipocyte-derived hormone encoded by ‘ob’ gene, has been proposed as the peripheral signal indicating the adequacy of nutritional status for reproductive functions.[4] But contradictory reports are available pertaining to how leptin modulates or is being modulated by pituitary gonadotropins or sex steroids. Polycystic ovarian syndrome (PCOS), a major form of dysovulatory infertility in women, is often associated with obesity and insulin resistance, both of which are features that are linked to leptin and its receptors. Serum level of leptin is higher in obese women. Obesity modifies insulin sensitivity and gonadotropin dynamics and is associated with disorders of ovulation. But, the relationship between leptin and insulin sensitivity, sex steroids, and insulin concentration in derangement of ovarian function still remain elusive and controversial.[5] It has been proposed that in part by increased intra-follicular levels of leptin, obesity directly affects ovarian functions in PCOS and may induce relative resistance to gonadotropins.[6] Taking into consideration the known association between leptin, obesity, and insulin action, it can be assumed that leptin might have a role in PCOS. Women with PCOS are characterized by hyperandrogenemia, increased leutinizing hormone (LH) concentrations, and high incidence of hyperinsulinemia/insulin resistance and obesity. Thus, PCOS patients may serve as a reliable model to assess the relationship of hyperinsulinemia and androgen excess with leptin concentrations beyond the association of leptin and obesity per se. Present study is, therefore, centered on the objective of evaluating the inter-relationship between serum leptin level with body mass index, insulin and with circulating testosterone in PCOS women.
Subjects and Methods
Study subjects
34 Indian women aged between 24-36 years who were otherwise healthy but complained of primary or secondary infertility lasting for 2-12 years and enrolled for IVF-ET were recruited in this study. The patients and their partners were thoroughly investigated (indices of ovulation, transvaginal ultrasonography, laparoscopy with assessment of tubal patency, semen analysis, and endocrine milieu) for the cause of infertility. All of them were euthyroid and normoprolactinemic. None of them had detectable pituitary or hypothalamic dysfunction. Moreover, none had received any drugs known to interfere with hormonal concentrations for at least 3 months before the study.
They were primarily grouped in to following categories:
i. Patients having infertility due to tubal block with no other pelvic abnormality and male factor infertility (includes healthy women with regular menstrual cycles of 26-30 days, normal-sized ovary and no sign of hyperandrogenism).
ii. PCOS. Diagnosis of PCOS was done according to the Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome.[7]
Women diagnosed with tubal block and male factor infertility were recruited as ‘control’ (n = 18) group while women with PCOS (n = 16) served as the study group.
Depending on BMI, both the control and the PCOS women were further categorized under obese (BMI >25) and non-obese (BMI ≤ 25) sub-groups. Informed consent was obtained from all patients prior to inclusion in the study. This study was conducted with the approval of the research ethics board of the institute.
Intervention
All were reviewed for their body mass index (BMI, Kg/m2), blood glucose, endocrine milieu [including baseline LH, follicle stimulating hormone (FSH), thyroid stimulating hormone (TSH), prolactin, estradiol (E2), testosterone, androstenedione, DHEA-S, and insulin] and for circulating serum leptin level.
Outcome measures
Fasting blood samples for the assessment of hormone profiles were collected either from spontaneous (in case of control patients) or from withdrawal (in case of PCOS patients by contraceptive challenge) cycles on 2nd day of menstruation. For PCOS patients, fasting blood samples were also obtained followed by a 75 gm, 2-hours oral glucose tolerance test (OGTT). All samples were obtained between 8:00 and 10:00. Sera were procured after centrifugation and stored at -40°C for the assay of hormones and for leptin.
Bio-chemical assays
All serum samples were assayed for leptin with immunoradiometric assay (IRMA; REF-DSL-23100; Diagnostic Systems Laboratories INC. 445 Medical Center Blvd; Webster, Tx, 77598, USA) with assay sensitivity of 0.10 ng/ml. Samples were analyzed for E2 (assay sensitivity: 9 pg/ml) using enzyme linked fluorescent assay (ELFA; VIDAS, Bio Merieux; Marcy I’Etoile, France). Samples were also evaluated for TSH (assay sensitivity 0.011 μIU/ml), LH (assay sensitivity 0.07 μIU/ml), FSH (assay sensitivity 0.3 μIU/ml), prolactin (assay sensitivity: 0.3 ng/ml) by a two-site chemiluminescence sandwich immunoassay system (Bayer Diagnostics Corporation, tarrytown, NY). Apart from this, radioimmunoassay (RIA) was used to assay serum testosterone (using commercial kit from Immunotech [BP, 177-13276; Marseille Cedex-9-France; REF-IM1119], with assay sensitivity of 0.025 ng/ml), dehydroepiandrosterone sulfate (DHEA-S, using the Biotecx DHEA-S Direct RIA kit [Ref. 210] with assay sensitivity of 10 ng/ml), and insulin (using commercial Kit from Diagnostic Products Corporation, Los Angeles, CA, USA with assay sensitivity of 1.3 μIU/ml) level. Measurement of androstenedione, on the other hand, was carried out through IMMULITE 2000-competitive chemiluminescent enzyme immunoassay with the assay sensitivity of 0.3 ng/ml. OGTT [8] was performed for the diagnosis of insulin resistance. Estimation of blood glucose was done by the glucose oxidase method. Fasting insulin: Glucose ratio (IGR, μIU/mg) was also calculated to determine insulin sensitivity.[9]
Statistical analysis
Results were expressed as mean ± standard error of mean (SE). Results of PCOS and control women were compared by group t-tests. Variables including LH and fasting levels of leptin and insulin were log10 transformed to achieve normality. Relationship between leptin and independent variables were evaluated by Pearson (r) correlation coefficients and stepwise multivariate linear regression. All statistical analyses were done using PRISM Statistical Software Package (PRISM Version 4.03@1992-2005; GraphPad Software Inc). P < 0.05 were considered significant.
Results
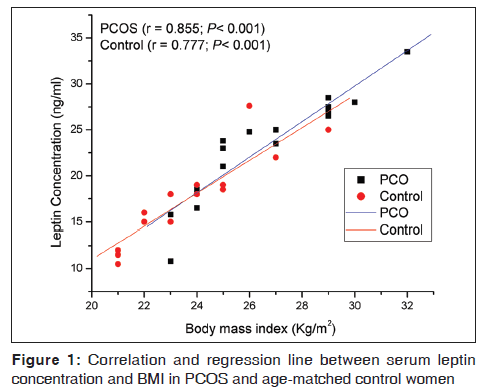
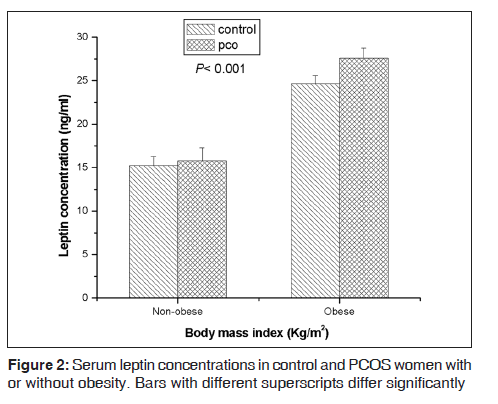
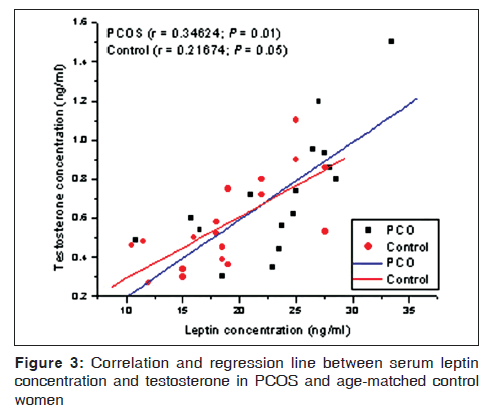
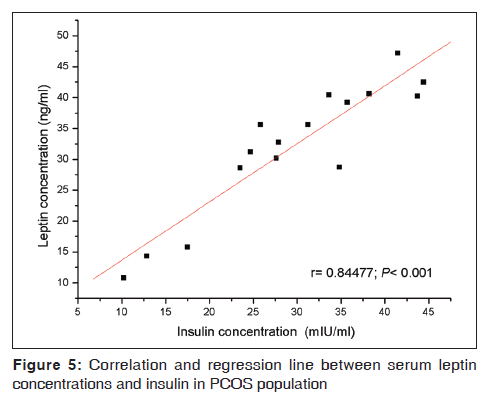
Both the study populations were age-matched [Table 1]. As compared with the control, the mean BMI, LH, and LH: FSH ratios were elevated in the PCOS population [Table 1]. The latter group also had significantly elevated levels of testosterone, androstenedione, and fasting levels of insulin [Table 1]. When serum leptin levels were analyzed as a function of BMI, linear regression analysis revealed a positive correlation between the two in both control as well as PCOS population [Figure 1]. However, leptin levels in a subgroup of PCOS women were found proportionately further elevated with respect to their corresponding BMI. Regularly cycling control women had significantly increased leptin levels if they were obese compared to lean [Figure 2]. Leptin concentrations in non-obese PCOS women were elevated compared to non-obese control women [Figure 2]. In obese PCOS women, the serum leptin levels were significantly higher than non-obese women with PCOS and all control women [Figure 2]. The mean serum leptin levels in PCOS women were elevated, but there was substantial overlap in the individual leptin levels of women with PCOS and the controls. A high proportion of women with PCOS were hyperandrogenic. The mean serum testosterone level in PCOS patients was significantly increased compared to control [Table 1]. But, when serum testosterone level was analyzed as a function of serum leptin, no significant correlation could be found between testosterone and leptin levels in either of the groups [Figure 3]. Baseline insulin levels were higher in women with PCOS than in controls [Table 1]. Of the 16 PCOS women, 6 women were diagnosed to have hyperinsulinemia (>27 μIU/mL of fasting insulin). When these 6 women were analyzed separately, it was observed that these women represent that subset of PCOS group who had proportionately elevated leptin relative to BMI. Insulin-resistant PCOS women had mean serum leptin levels >2-fold higher than controls. A positive correlation was found between leptin levels and insulin both in the control [Figure 4] and PCOS [Figure 5] population.
| Study parameters | Control(n=18) | PCOS(n=16) | P |
|---|---|---|---|
| Age (years) | 28.28 (0.87) | 27.92 (0.96) | 0.78 |
| BMI (Kg/m2) | 24.52 (0.73) | 27.51 (0.74) | <0.01 |
| TSH (μIU/mL) | 2.85 (0.40) | 2.78 (0.38) | 0.89 |
| Prolactin (ng/ml) | 13.26 (1.35) | 12.40 (1.40) | 0.65 |
| LH (IU/L) | 5.28 (0.35) | 8.94 (0.89) | <0.001 |
| FSH (IU/L) | 5.79 (0.31) | 4.44 (0.22) | <0.01 |
| LH: FSH ratio | 0.91 (0.26) | 2.00 (0.28) | <0.01 |
| Estradiol (pg/mL) | 38.28 (3.76) | 47.15 (7.48) | 0.27 |
| Testosterone (ng/mL) | 0.41 (0.11) | 1.02 (0.13) | <0.001 |
| Androstenedione (ng/mL) | 1.62 (0.25) | 2.96 (0.28) | <0.01 |
| DHEA-S (ng/mL) | 1650 (300) | 2476 (450) | 0.12 |
| Fasting insulin (μIU/mL) | 13.71 (1.47) | 23.86 (2.20) | <0.001 |
| Fasting leptin (ng/mL) | 16.86 (0.90) | 29.20 (0.70) | <0.001 |
PCOS: Polycystic ovarian syndrome, BMI: Body mass index, TSH: Thyroid stimulating hormone, LH: Leutinizing hormone, FSH: Follicle stimulating hormone, DHEA-S: Dehydroepiandrosterone sulfate
Table 1: Clinical and endocrine characteristics of control and PCOS women
Discussion
Ovary is an ever-changing tissue and dynamic multicompartmental organ, which is under the chief regulatory control of hypothalamic, pituitary principles. The hypothalamic-pituitary control over ovarian functions, however, is precisely governed by a plethora of external factors and internal peripheral principles including many of ovarian origin. Leptin has emerged as peripheral signal and a potential regulator of many reproductive functions including gametogenic and steroidogenic potential of ovary. It is considered as a possible link between nutrition and reproduction.[10] Reproductive potential in women undergoes adverse alteration following severe changes in nutritional status and energy availability in both directions. These adaptive changes are reversible when nutritional status is normalized.[11,12] Leptin is important in regulating energy homeostasis, and by this virtue impacts the reproductive systems in diverse ways.[13] Leptin concentration shows fluctuations during menstrual cycle,[14] which indicates that in adult women, possibly a feedback interaction exists between leptin and the hypothalamo-pituitary-ovarian (HPO) axis. Moreover, leptin receptor and mRNA have been identified in the HPO axis.[15,16] Leptin mRNA and protein production have also been documented in granulosa cells, oocytes, and early cleavage stage embryos.[17] Expression of leptin receptors in granulosa cells, which synergies with glucocorticoids to promote steroidogenesis, indicates that leptin exerts direct regulatory action in ovarian folliculogenesis.[18] Furthermore, leptin has been shown to regulate expression of mRNAs for side chain cleavage enzyme and 17a-hydroxylase [19] and to modulate LH-stimulated estradiol production [20] in the ovary. Serum leptin is found to be keenly interrelated with estrogens, progesterone, androgens, and insulin. But, their role in the regulation of circulating leptin levels is still obscure. Though leptin is widely present in reproductive tissues, its relationship to reproductive hormones is still poorly understood. Controversial results have been reported during hormone replacement therapy, oral contraceptive intake, and ovulatory disorders.[21,22] Polycystic ovarian syndrome, the common dysovulatory infertility, is characterized by chronic anovulation, hyperandrogenemia, insulin resistance, and a high incidence of obesity;[7] features which are often linked to leptin and its receptor. These facts thus make PCOS women a useful tool to assess the inter-regulatory phenomena between leptin and ovarian function. The results of the present study confirm that a positive correlation exists between serum leptin and BMI. Unlike the results of Rouru, et al.[23] and Laughlin, et al.[24] that leptin levels were similar in both PCOS patients and control subjects, the major finding of the present investigation is that, irrespective of BMI or insulin resistance, PCOS population has higher leptin levels. This observation is quite legitimate, because leptin is predominantly synthesized by adipocytes, and higher BMI is distributed with high frequency in the PCOS group than in control women. The difference in the leptin levels reached greater magnitude of significance when PCOS women were categorized under obese (BMI > 25) and non-obese (BMI ≤ 25) sub-groups. Moreover, the most noteworthy of all is to observe that even after normalization of most of the known confounding factors, the PCOS women tended to have increased serum levels of leptin. Especially, a subset of PCOS population had significantly elevated leptin level even after the adjustment of BMI. This finding of hyperleptinemia in PCOS appears to be a masking effect of hyperinsulinemia after transformation of data to achieve normality to account for the distribution of insulin resistance (IR) between the PCOS and the control groups. Leptin levels were found statistically comparable between the two groups having comparable magnitude of BMI and IR. Within the PCOS group, normalization of data in respect of obesity and IR, however, showed positive influence of insulin and obesity individually on fasting leptin levels, independent of adiposity and IR, respectively. Insulin has been shown to increase leptin mRNA in adipocytes, suggesting its possible role in stimulating leptin secretion.[25] It may be that the elevated leptin in hyperinsulinemic PCOS women is a secondary consequence of insulin-stimulated synthesis of leptin. Leptin on the other hand, inhibits insulin-mediated promotion of gonadotropin-stimulated steroidogenesis. There are reports that leptin decreases glucose-mediated insulin secretion through its receptors in the hypothalamus and also attenuates its action at the cellular level.[26] The cellular signaling interactions among gonadotropins, insulin, and leptin thus appear very complex. Furthermore, in this investigation, a high proportion of women with PCOS were found hyperandrogenic. The mean baseline serum testosterone level in PCOS patients was found significantly higher compared to control. But, when serum testosterone was analyzed as a function of serum leptin, no significant correlation could be found between testosterone and leptin in either of the groups. High leptin levels, although was theoretically likely to inhibit ovarian steroidogenesis in PCOS women, elevated leptin levels are associated with elevated testosterone levels. Therefore, there must be some as yet undefined mechanism underlying insulin-mediated ovarian androgen production.
Taken together the present observations made in this study propose that regulation of leptin’s synthesis and its action on female reproductive system are governed by a plethora of controlling factors. Small size of the study population limits the statistical power to judge precise correlation; however, elevated serum leptin in polycystic ovarian women appears to be due to the positive correlation between leptin and BMI and insulin levels. The observations presented herein should be viewed as a prelude to what future holds.
Acknowledgment
This study was supported financially partly by University Grants Commission, New Delhi, India.
Source of Support: Financially partly supported by the University Grants Commission, New Delhi, India.
Conflict of Interest: None declared.
References
- Tataranni PA, Monroe MB, Dueck CA, Traub SA, Nicolson M, Manore MM, et al. Adiposity, plasma leptin concentration and reproductive function in active and sedentary females. Int J Obes Relat Metab Disord 1997;21:818-21.
- Miller KK, Parulekar MS, Schoenfeld E, Anderson E, Hubbard J, Klibanski A, et al. Decreased leptin levels in normal weight women with hypothalamic amenorrhea: The effects of body composition and nutritional intake. J Clin Endocrinol Metab 1998;83:2309-12.
- Chu NF, Shen MH, Wu DM, Shieh SM. Plasma TNF-R1 and insulin concentrations in relation to leptin levels among normal and overweight children. Clin Biochem 2002;35:287-92.
- Almog B, Gold R, Tajima K, Dantes A, Salim K, Rubinstein M, et al. Leptin attenuates follicular apoptosis and accelerates the onset of puberty in immature rats. Mol Cell Endocrinol 2001;183:179-91.
- Maliqueo M, Pérez-Bravo F, Calvillán M, Piwonka V, Castillo T, Sir-Petermann T. Relationship between leptin and insulin sensitivity in patients with polycystic ovary syndrome. Med Clin (Barc) 1999;113:526-30.
- Pinilla L, Seoane LM, Gonzalez L, Carro E, Aguilar E, Casanueva FF, et al. Regulation of serum leptin levels by gonadal function in rats. Eur J Endocrinol 1999;140:468-73.
- Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. The androgen excess and PCOS society criteria for the polycystic ovary syndrome: The complete task force report. Fertil Steril 2009;91:456-88.
- World Health Organization and International Diabetes Federation. Definition, diagnosis and classification of diabetes mellitus and its complications. Geneva, Switzerland: World Health Organization; 1999.
- Spritzer PM, Poy M, Wiltgen M, Mylius LS, Capp E. Leptin concentrations in hirsute women with polycystic ovary syndrome or idiopathic hirsutism: Influence on LH and relationship with hormonal, metabolic, and anthropometric measurements. Hum Reprod 2001;16:1340-46.
- Clark AM, Ledger W, Galletly C, Tomlinson L, Blaney F, Wang X, et al. Weight loss results in significant improvement in pregnancy and ovulation rates in anovulatory obese women. Hum Reprod 1995;10:2705-12.
- Lonnqvist F, Arner P, Nordfors L, Schalling M. Overexpression of the obese (ob) gene in adipose tissue of human obese subjects. Nat Med 1995;1:950-3.
- Rogers J, Mitchell GW Jr. The relation of obesity to menstrual disturbances. N Engl J Med 1952;247:53-5.
- Kaseki H, Maruyama S, Ishihara K, Araki T. Serum leptin concentration in young adult women with ovulatory dysfunction. J Nihon Med Sch 2003;70:270-73.
- Hardie L, Trayhurn P, Abramovich D, Fowler P. Circulating leptin in women: A longitudinal study in the menstrual cycle and during pregnancy. Clin Endocrinol (Oxf) 1997;47:101-6.
- Brannian JD, Hansen KA. Leptin and ovarian folliculogenesis: Implications for ovulation induction and ART outcomes. Semin Reprod Med 2002;20:103-12.
- Cioffi JA, Van Blerkom J, Antczak M, Shafer A, Wittmer S, Snodgrass HR. The expression of leptin and its receptors in pre-ovulatory human follicles. Mol Hum Reprod 1997;3:467-72.
- Finn PD, Cunningham MJ , Pau KY, Spies HG, Clifton DK, Steiner RA. The stimulatory effect of leptin on the neuroendocrine reproductive axis of the monkey. Endocrinology 1998;139:4652-62.
- Sir-Petermann T, Maliqueo M, Palomino A, Vantman D, Recabarren SE, Wildt L. Episodic leptin release is independent of luteinizing hormone secretion. Hum Reprod 1999;14:2695-99.
- Zamorano PL, Mahesh VB, De Sevilla LM, Chorich LP, Bhat GK, Brann DW. Expression and localization of the leptin receptor in endocrine and neuroendocrine tissues of the rat. Neuroendocrinology 1995;65:223-8.
- Karlsson C, Lindell K, Svensson E, Bergh C, Lind P, Billig H, et al. Expression of functional leptin receptors in the human ovary. J Clin Endocrinol Metab 1997;82:4144-8.
- Brannian JD, Schmidt SM, Kreger DO, Hansen KA. Baseline non-fasting serum leptin concentration to body mass index ratio is predictive of IVF outcomes. Hum Reprod 2001;16:1819-26.
- Carmina E, Ferin M, Gonzalez F, Lobo RA. Evidence that insulin and androgens may participate in the regulation of serum leptin levels in women. Fertil Steril 1999;72:926-31.
- Rouru J, Anttila L, Koskinen P, Penttila TA, Irjala K, Huupponen R, et al. Serum leptin concentrations in women with polycystic ovarian syndrome. J Clin Endocrinol Metab 1997;82:1697-700.
- Laughlin GA, Morales AJ, Yen SS. Serum leptin level in women with polycystic ovary syndrome: The role of insulin resistance/ hyperinsulinemia. J Clin Endocrinol Metab 1997;82:1692-6.
- Vidal H, Auboeuf D, De Vos P, Staels B, Riou JP, Auwerx J, et al. The expression of ob gene is not actually regulated by insulin and fasting in human abdominal subcutaneous adipose tissue. J Clin Invest 1996;98:251-5.
- Dagogo-Jack S, Fanelli C, Paramore D, Brothers J, Landt. M. Plasma leptin and insulin relationships in obese and non-obese humans. Diabetes 1996;45:695-8.





 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.