Systemic Lupus Erythematosus presenting as Spinal Cord Infarction and Vertebral Artery Aneurysm: A Case Report
2 Department of Internal Medicine, Cardinal Santos Medical Center, San Juan City, Philippines, Email: audreychua17@gmail.com
3 Department of Clinical Neurosciences, University of the East Ramon Magsaysay Memorial Medical Center, Quezon City, Philippines
Received: 05-May-2023, Manuscript No. amhsr-23-97917; Editor assigned: 08-May-2023, Pre QC No. amhsr-23-97917 (PQ); Reviewed: 23-May-2023 QC No. amhsr-23-97917; Revised: 30-May-2023, Manuscript No. amhsr-23-97917 (R); Published: 06-Jun-2023
Citation: Audrey Marie UC, et al. Systemic Lupus Erythematosus presenting as Spinal Cord Infarction and Vertebral Artery Aneurysm: A Case Report. Ann Med Health Sci Res. 2022;13:554
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Spinal cord infarction has been reported to be a rare disease comprising of only 1% to 2% of all ischemic strokes. The finding of a dissecting vertebral artery aneurysm combined with positive for Antinuclear Antibodies (ANA) presenting at first as a Spinal Cord Infarction (SCI) is a unique case of a Central Nervous System (CNS) Systemic Lupus Erythematosus (SLE). We report a case of a 54-year-old female from the Philippines who presented with bilateral lower extremity weakness and urinary incontinence. Magnetic Resonance Imaging (MRI) of the thoracolumbar spine revealed focal intrinsic signal abnormalities, indicative of restricted diffusion, involving T11 midbody down to the apex of the conus, consistent with an acute infarction. Cranial Computed Tomography (CT) angiography also showed a sacculo-fusiform aneurysm at the junction of the 3rd and 4th segment of the left vertebral artery with probable dissection, respectively. The patient was also noted to have increased Cerebrospinal Fluid (CSF) Immunoglobulin G (IgG) and positive for ANA.
Keywords
Spinal cord infarction; Vasculitis; COVID-19; SARS-COV-2; Lupus erythematosus; Vertebral artery dissection; Myelitis; Myelopathy; Subarachnoid Hemorrhage (SAH)
Introduction
SCI is a rare disease that widely varies in its clinical presentations and vertebral artery dissection can be seen in 4% to 10% of SCI. These has left patients with harmful neurological outcomes, such as paraplegia, quadriplegia, and urinary incontinence [1,2]. The spinal cord receives a vascular supply which consists of two arterial systems that form a rich anastomotic network, the single anterior spinal artery and paired posterior spinal arteries in which the former supplies the anterolateral two-thirds of the spinal cord, and the latter supplying the posterior one-third of the spinal cord making the spinal cord relatively resistant to ischemia [3]. SCI develops when the vascular supply to the spinal cord fails and typical causes of infarct include nonprocedural and procedural etiologies such as cardio-embolism, fibrocartilaginous embolism, arterial dissections and systemic hypotension but can also develop as a complication after endovascular, aortic or vertebral artery procedures [3]. Subarachnoid Hemorrhage (SAH) complication secondary to arterial dissection can be associated with trauma or spontaneously while artery-to-artery embolic events are the most common mechanism of stroke, followed by hemodynamic failure [4]. The cause of the aneurysm could be a combination of vasculitic weakening of the vessel wall from pro-inflammatory manifestations of SLE and possible dilation of the vertebral artery. The following case report serves to illustrate the key points of this condition and discuss the possible mechanisms leading to the SCI and subarachnoid hemorrhage in patients with SLE.
Case Presentation
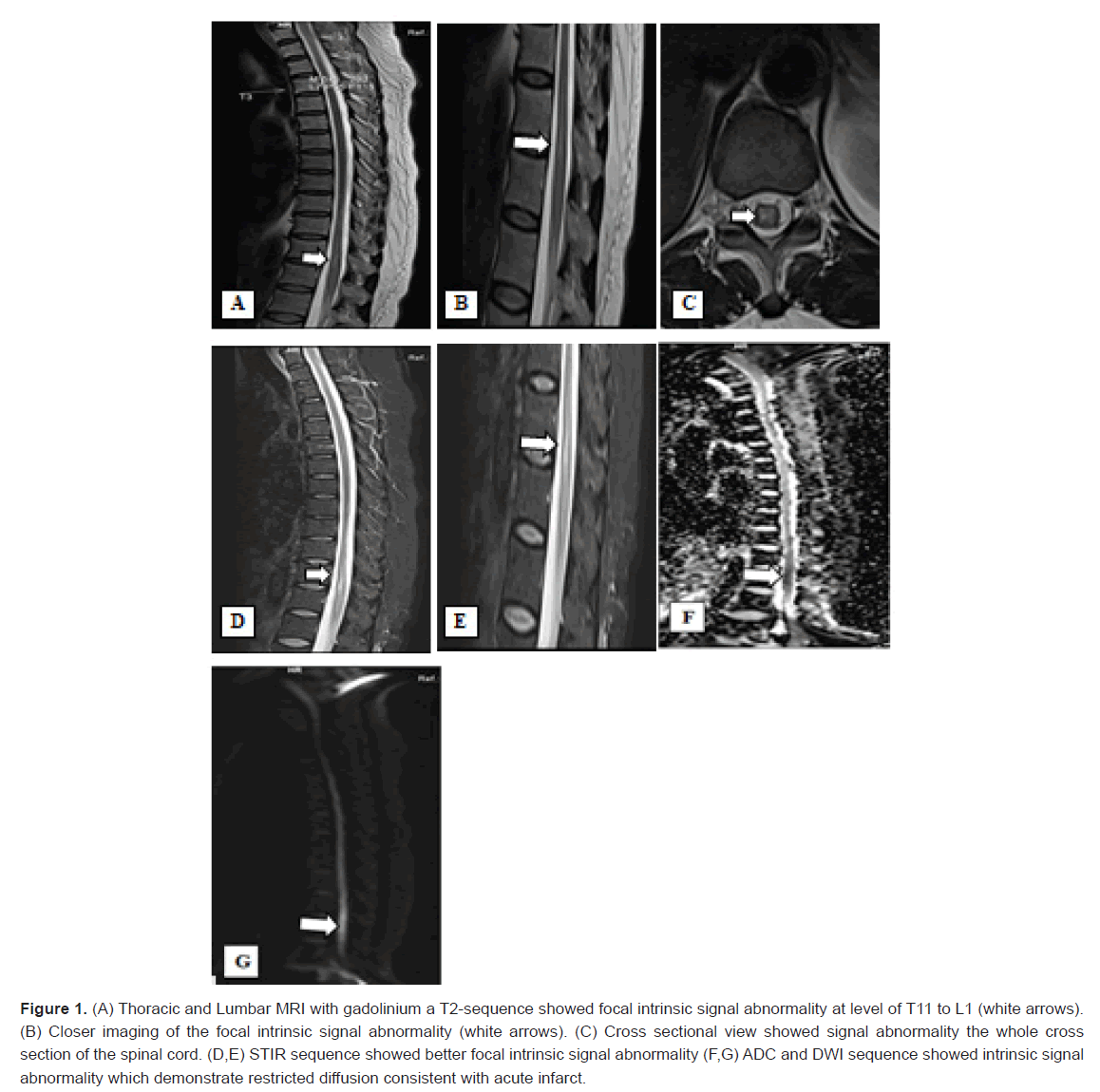
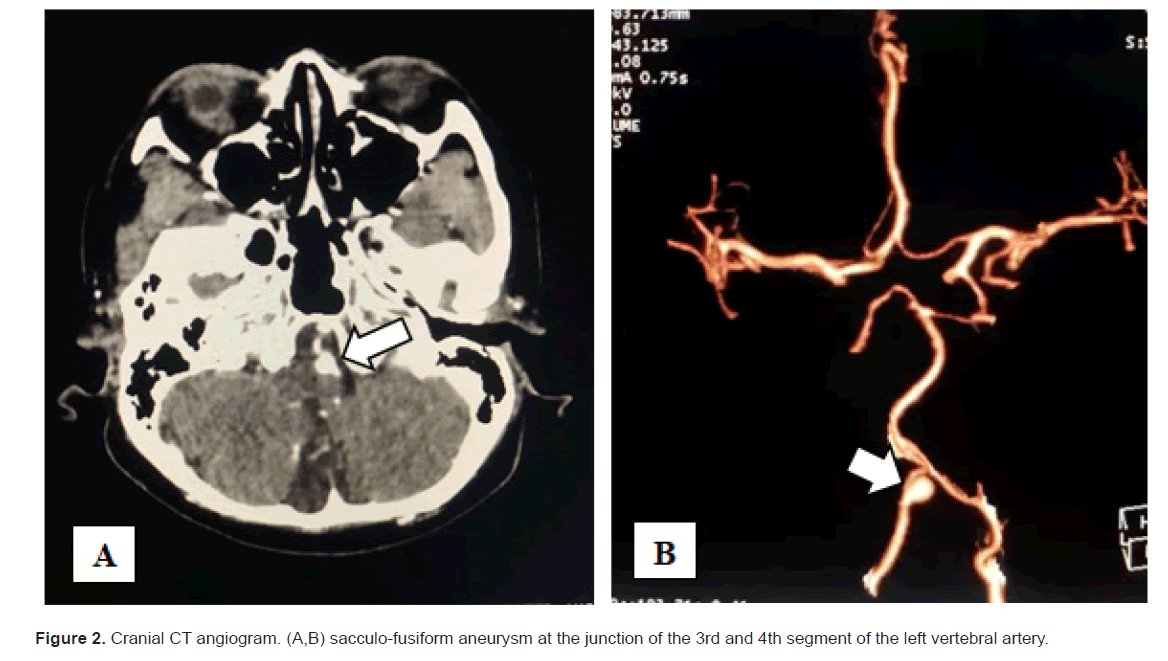
We present a 54-year-old female, who had mild COVID-19 infection three weeks prior to admission and presented initially at the emergency room with bilateral lower extremity weakness and decreased sensation at the level of L4 and below as well as urinary bladder distention. Other pertinent findings on physical examination showed erythematous non pruritic rashes over the neck, face, dorsal and palmar surfaces of the hands. Patient underwent thoracic and lumbar MRI with gadolinium contrast and showed a restricted diffusion involving the midbody of T11 down to the conus tip consistent with an acute infarct with the main consideration of cord ischemia that cannot exclude edema or inflammation (Figure 1). Patient was initially treated as a case of spinal shock secondary to post infective acute transverse myelitis and was then initially started on Methylprednisolone 1 gram per IV once daily for five days. After the first day of methylprednisolone treatment, patient noted slight improvement of the motor weakness and sensory deficit of the bilateral lower extremities. Lumbar tap was done and CSF analysis revealed an opening pressure of 16 cm H20 and closing pressure of 12 cm H20. There is slight elevation of protein at 0.71 g/L and increased Red Blood Cells (RBC) count of 2,169 × 10^9/L. A serial RBC was subsequently requested which showed increasing RBC trends of bottle 1: 140 × 10^9/L, bottle 2: 750 × 10^9/L and bottle 3: 2,169 × 10. Additional work up for autoimmune disease such as CSF IgG and CSF oligoclonal bands and serum IgG and ANA was requested as well. CT angiogram was done to rule out ruptured aneurysm, which revealed a sacculo-fusiform aneurysm at the junction of the 3rd and 4th segment of the left vertebral artery measuring 4.5 × 5.7 mm (L × W), and its neck measuring 6.0 mm with probable dissection (Figure 2). She was referred to Neurosurgery service and was advised a 4-vessel angiography with possible coiling. However, the patient decided to transfer to another hospital to facilitate the procedure. Laboratory tests sent were negative for oligoclonal bands but positive for serum ANA in a 1:40 spot pattern and positive for CSF IgG at 16 mg/dL, suggesting an autoimmune process. The patient was suspected to have heavy systemic inflammation and with these findings, an underlying autoimmune disease such as SLE myelitis was considered. The patient was discharged for transfer to another institution for further work-up and management.
Figure 1: (A) Thoracic and Lumbar MRI with gadolinium a T2-sequence showed focal intrinsic signal abnormality at level of T11 to L1 (white arrows). (B) Closer imaging of the focal intrinsic signal abnormality (white arrows). (C) Cross sectional view showed signal abnormality the whole cross section of the spinal cord. (D,E) STIR sequence showed better focal intrinsic signal abnormality (F,G) ADC and DWI sequence showed intrinsic signal abnormality which demonstrate restricted diffusion consistent with acute infarct.
On follow-up, patient underwent endovascular coiling with stent insertion of the left vertebral artery in a different hospital two months after the incident. A plain thoraco-lumbar MRI post procedure was requested that showed signal abnormality involving the spinal cord at T11 vertebral body level down to the conus tip consistent with spinal cord ischemia in comparison with the contrast thoraco-lumbar MRI done in our institution. The cerebral angiography was not repeated postoperatively. She was advised to undergo work-up to rule out autoimmune disease or rheumatologic cause of aneurysm but did not proceed due to personal preference not to undergo further tests. Patient had at least 10 sessions of physical therapy and eventual progressive improvement of symptoms were observe after a month. Patient is now able to walk few steps with a walker and swimming with assistance 9 months after the procedure.
Results and Discussion
SCI is a rare disease accounting for only 1% of all strokes while vertebral artery dissection can be seen in 4% to 10% of SCI. SCI can most often induced by procedural related etiology such as aortic surgery, vertebral angiography, renal artery embolization, and intra-aortic balloon counterpulsation [5]. Nonprocedural related causes of SCI include embolism, arterial dissection, atherosclerosis, vasculitis, systemic hypotension, vascular malformations and hypercoagulable states while the remaining cause of SCI cases remained idiopathic [4].
SLE is a chronic autoimmune disease that affects multiple organ systems and myelopathy is one of the rare neuropsychiatric syndromes related to SLE defined by the American College of Rheumatology (ACR) accounting for 1%-2% of patients, and it is usually present as a severe manifestation of motor deficits as well as sensory deficits, and urinary dysfunction. Spinal cord involvement in SLE can be due to demyelination, thrombosis and vasculitis as well as infective and compressive causes [6-8]. Myelitis seldom occurs and can be the first manifestation of SLE [7]. Diagnosis of SLE myelitis is based on clinical findings, laboratory tests and the use of gadolinium-enhanced magnetic resonance imaging [8]. Cerebrovascular manifestation of SLE have been attributed to either vasculopathy or thrombosis as well as vasculitis of small arteries or arterioles [5]. Ischemia holds to be the main cause of CNS manifestation in SLE. The mechanism leading to ischemia is diverse and involve predisposition in abnormalities of coagulation, thickening of vessel walls due to different causes, the development of atherosclerotic plaques in large arteries and immune complex deposition and inflammatory cases as well [9,10].
Subarachnoid hemorrhage due to ruptured aneurysm is uncommon in SLE patients wherein the incidence rate in North America and Europe is approximately 0.1% [8]. There are few studies and reports that identified histopathologic inflammation, vasculitis, and fibrinoid necrosis within ruptured aneurysm walls present in patients with SLE [10]. Hence, inflammation can also induce endothelial remodeling causing aneurysmal growth increasing the risk for the rupture of aneurysm [10]. Other pathophysiology of subarachnoid hemorrhage in patients affected with SLE include: (1) those with classical risk factors such as hypertension and atherosclerosis that is a complication of treatment with steroids or cytotoxic immunosuppressants; (2) vasculitic changes in which there is an inflammation of the arteries and narrowing the arterial lumen thus compromising vascular or cerebral blood flow leading to ischemia or hemodynamic stress [10,11].
In this case report, the SCI can be caused by different etiologies such as in a form of vertebral artery dissection causing hypoperfusion over the T11 area or an embolism formed from the dissection. Vasculitis was also entertained, which could be due to the systemic inflammation brought about by SLE. The patient’s condition, being in a pro-inflammatory state due to SLE, increased her chance of having a vasculitic stroke with complications, such as an acute ischemic stroke, subarachnoid hemorrhage, or intracerebral hemorrhage. The neuroimaging seen as T2 weighted hyperintense signal with high diffusion weighted imaging and low ADC signal consistent with that of an acute infarct.
Differential diagnosis includes post-infective acute transverse myelitis, multiple sclerosis and neuromyelitis optica spectrum disorder. These were excluded based on history, examination, and diagnostic workup. With this unique case presented by the patient and diagnostic procedures did rule in the finding of an acute spinal cord infarction secondary to vertebral artery dissection brought about by SLE. The findings of a characteristic spinal cord lesion in our patient would support a mechanism caused by SLE.
Our study has several limitations. We were not able to request for other autoimmune markers such as anti-dsDNA, SS-A, SSB, ANCA and antiphospholipid antibodies. Further diagnostic investigations to completely rule out demyelinating diseases and ideally, a Cranial MRI and Aquaporin-4 anitbody or NMOIgG should be performed.
Conclusion
SLE is rarely associated with vertebral aneurysm, subarachnoid hemorrhage, or spinal cord infarction. This case is uncommon in patients with SLE and is an important manifestation of neuropsychiatric involvement in SLE. Findings of myelitis are no longer included in the new clinical neurological criteria for the European League Against Rheumatism (EULAR)/ American College of Rheumatology (ACR) 2019 classification of SLE comparing that with the Systemic Lupus International Collaborating Centers (SLICC) criteria 2017 due to its low sensitivity, but this case report can serve as an important finding that could support that myelitis could still be a manifestation in neuropsychiatric criteria for SLE and may be the first manifestation of the disease. The report will provide new insights into neurologists and rheumatologists as well as cases of myelitis in SLE patients.
References
- Hsu JL, Cheng MY, Liao MF, Ching Hsu H, Weng YC, et al. The etiologies and prognosis associated with spinal cord infarction. Ann Clin Transl Neurol. 2019;6:1456-1464.
[Crossref], [Google Scholar], [PubMed]
- Hanna Al-Shaikh R, Czervionke L, Eidelman B, Dredla BK. Spinal cord infarction. In: StatPearls. Treasure Island (FL): StatPearls Publishing. 2022.
- Sarto J, Semerano A, Moreno JL, Mayà-Casalprim G, Blasco J, et al. Spinal cord hemodynamic infarction after vertebral artery endovascular trapping despite preserved flow in the anterior spinal artery. J Spinal Cord Med. 2021;44:1001-1004.
[Crossref], [Google Scholar], [PubMed]
- Montalvo M, Bayer A, Azher I, Knopf L, Yaghi S. Spinal cord infarction because of spontaneous vertebral artery dissection. Stroke. 2018;49:e314-e317.
[Crossref], [Google Scholar], [PubMed]
- Mimori A, Suzuki T, Hashimoto M, Nara H, Yoshio T, et al. Subarachnoid hemorrhage and systemic lupus erythematosus. Lupus. 2000;9:521-526.
[Crossref], [Google Scholar]
- Chiganer EH, Hryb JP, Carnero Contentti E. myelitis and lupus: Clinical manifestations, diagnosis and treatment. Review. Mielitis y lupus: clínica, diagnóstico y tratamiento. Revisión. Reumatol Clin. 2017;13:344-348.
[Crossref], [Google Scholar], [PubMed]
- Mehta P, Gupta L, Muhammed H, Misra DP, Lawrence A, et al. Spectrum of myelitis in systemic lupus erythematosus: experience from a single tertiary care centre over 25 years. Mediterr J Rheumatol. 2021;32:31-38.
[Crossref], [Google Scholar], [PubMed]
- Zhang Y, Liu SF, Zeng XJ. Subarachnoid hemorrhage due to systemic lupus erythematosus associated with multiple intracranial artery aneurysms. Chin Med J. 2019;132:109-112.
[Crossref], [Google Scholar], [PubMed]
- Jennekens FG, Kater L. The central nervous system in systemic lupus erythematosus. Part 2. Pathogenetic mechanisms of clinical syndromes: A literature investigation. Rheumatology. 2002;41:619-630.
[Crossref], [Google Scholar], [PubMed]
- Graffeo CS, Tanweer O, Nieves CF, Belmont HM, Izmirly PM, et al. Rapid aneurysm growth and rupture in systemic lupus erythematosus. Surg Neurol Int. 2015;6:9.
[Crossref], [Google Scholar], [PubMed]
- Torné R, Rodríguez-Hernández A, Bernard T, Arikan Abelló F, Vilalta Castan J, et al. Subarachnoid hemorrhage in systemic lupus erythematosus: Systematic review and report of three cases. Clin Neurol Neurosurg. 2015;128:17-24.
[Crossref], [Google Scholar], [PubMed]




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.