The Complexity of Stromal Changes in Desmoplastic Ameloblastoma
- *Corresponding Author:
- Dr. Simranjit Singh
Department of Oral Pathology and Microbiology, Dr. Harvansh Singh Judge Institute of Dental Sciences and Hospital, Panjab University, Chandigarh - 160 014, India.
E-mail: drsmrnjt4@gmail.com
Abstract
Ameloblastoma is usually considered a homogenous neoplasm and is thought of as the most primitive of all odontogenic neoplasms. However, detailed investigations have proven clinicopathological diversity in a significant number of cases, thus mounting the evidence in favor of considering ameloblastoma as a mysterious lesion. The purpose of this article is to report a unique case of desmoplastic ameloblastoma and to throw light on the atypical changes noticed in the stromal component. The findings of this case have served to add interesting parameters to the study of stromal changes associated with this perplexing odontogenic tumor.
Keywords
Ameloblastoma, Jaw tumors, Odontogenic tumors, Stromal changes
Introduction
The ameloblastoma constitutes a puzzling paradox and is perhaps the most enigmatic of all odontogenic neoplasms.[1] Although ameloblastoma is generally regarded as a homogenous group of neoplasms, but clinicopathological diversity has been reported in a significant number of tumors, hence emphasizing the differentiation potential of neoplastic odontogenic epithelium.[2] Here we report a unique case of desmoplastic ameloblastoma involving the entire left maxillary sinus and presenting with a multifaceted histopathological picture.
Case Report
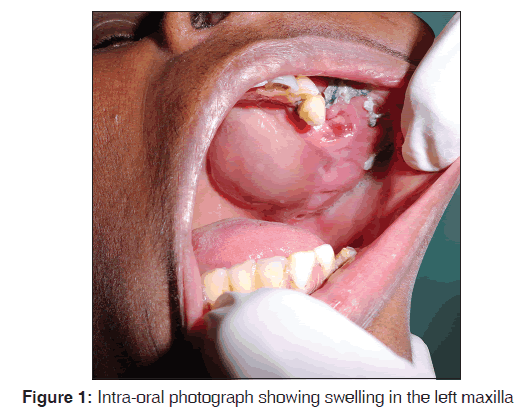
This was a case report of 45-year-old female patient who presented with a complaint of a painful enlarging swelling, 9 cm × 8 cm in size, of 2 years duration in the left maxillary region. On palpation, there was significant buccal and lingual cortical expansion [Figure 1].
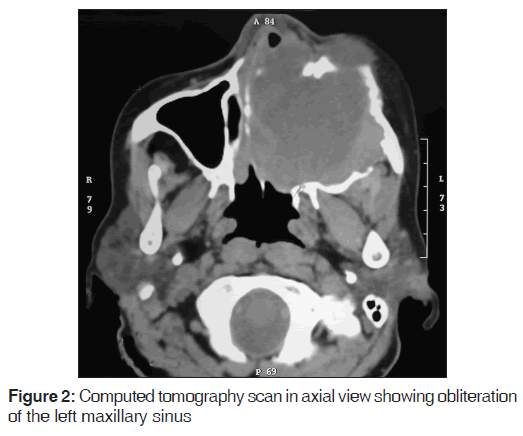
Orthopantomograph revealed a mixed radiolucent and radiopaque lesion extending from 21 region to the region of left maxillary tuberosity, superiorly it was extending up to the left infraorbital margin with haziness observed in the left maxillary sinus; the floor of the left orbit was intact. Computed tomography (CT) scan in axial view revealed a hyperdense mass involving the entire left maxillary sinus and breaching all the walls of the sinus [Figure 2].
Based on the clinical, radiological and the CT scan features, a provisional diagnosis was given as benign odontogenic tumor probably ameloblastoma. The incisional biopsy was reported as desmoplastic ameloblastoma based on which the patient underwent surgical excision of the mass under general anesthesia.
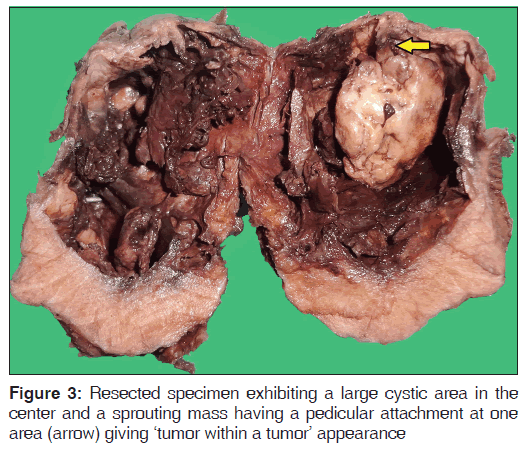
Macroscopically, the resected mass was grayish-white in color; measured 6 × 5.5 × 4.5 cm, was oval, had a lobulated surface and was firm in consistency. The specimen was cut in the middle and the features were examined. It showed a large cystic area in the center with sprouting mass having a pedicular attachment at one area. The outer area appeared to be solid in nature [Figure 3].
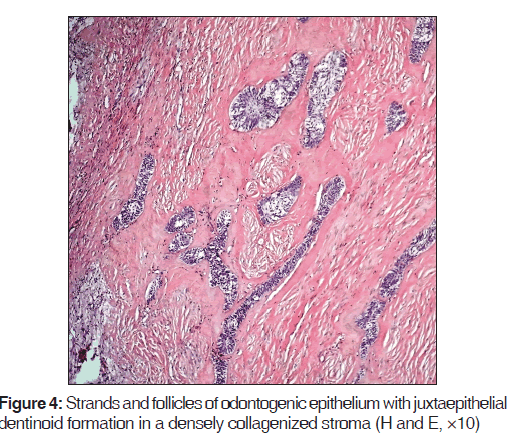
Microscopically, the lesion gave the picture of desmoplastic ameloblastoma in the form of odontogenic epithelium seen as follicles as well as strands simulating cord-like structures in a fibrous stroma. Peripheral columnar ameloblast-like cells were inconspicuous about the epithelial islands; however, in the areas where follicles showed expansion, well-formed peripheral columnar cells with reversed polarity were appreciated. The stromal component was densely collagenized giving a hyalinized picture [Figure 4].
An interesting finding was the presence of abundant eosinophilic, homogenous extracellular material juxtaepithelially. This material was interpreted as dentinoid [Figure 4].
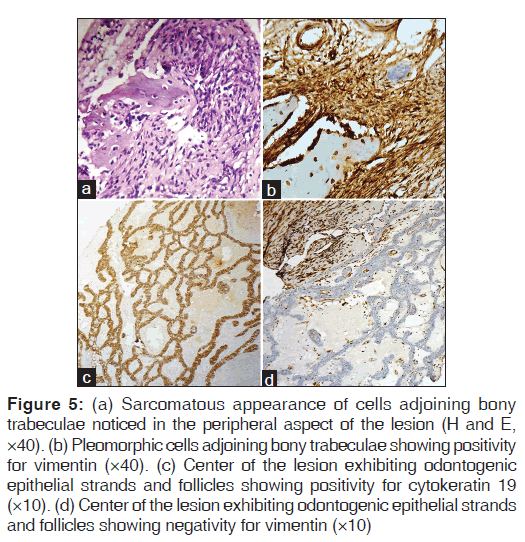
In the peripheral areas of the lesion, plump fibroblasts as well as pleomorphic cells invading the bony trabeculae, were noticed [Figure 5a]. These dysplastic cells were negative for cytokeratin 19 and positive for vimentin [Figure 5b], thus reflecting their mesenchymal nature. However, the center of the lesion exhibited odontogenic epithelial strands and follicles which were positive for cytokeratin 19 [Figure 5c] and negative for vimentin [Figure 5d].
Figure 5: (a) Sarcomatous appearance of cells adjoining bony trabeculae noticed in the peripheral aspect of the lesion (H and E, ×40). (b) Pleomorphic cells adjoining bony trabeculae showing positivity for vimentin (×40). (c) Center of the lesion exhibiting odontogenic epithelial strands and follicles showing positivity for cytokeratin 19 (×10). (d) Center of the lesion exhibiting odontogenic epithelial strands and follicles showing negativity for vimentin (×10)
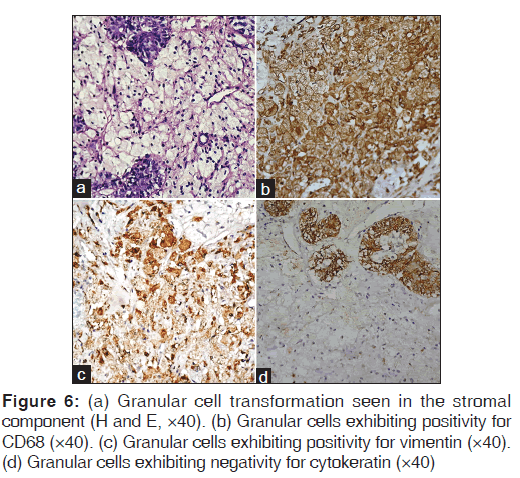
Another atypical finding was the presence of polygonal cells with abundant granular eosinophilic cytoplasm and peripherally placed nucleus [Figure 6a]. These cells were positive for CD68 [Figure 6b] and vimentin [Figure 6c] and were negative for cytokeratin [Figure 6d], thus confirming the stromal nature of the granular cells.
Discussion
Ameloblastoma is perhaps the most perplexing of all odontogenic neoplasms due to its unexplainable clinical and histopathological behavior. Because desmoplastic variant differs strikingly from other forms of ameloblastomas, it is considered as a separate clinicopathologic entity.[3] The present case of discussion matches with the clinical and radiological features as in previous literature.
Histologically desmoplastic variant exhibits irregular epithelial tumor islands being compressed by stromal component. The epithelial cells at the periphery of the islands are cuboidal with occasional hyperchromatic nuclei. Columnar cells with nuclear polarity are rarely conspicuous.[4] Our case exhibited similar features histopathologically.
The most intriguing paradox linked to ameloblastomas is the fact that, despite its name, no calcified dental tissue is formed in the interior of this tumoral mass. The possible explanation for this observation is that, in ameloblastoma, the environmental conditions are totally different as compared to normal odontogenesis. Thus the normal phenomenon of odontoblast differentiation under inductive influence of pre-ameloblasts, with subsequent formation of dentin does not occur because a mesenchyme capable of responding to this inductive action of the pre-ameloblasts does not exist; instead, a dense fibrous connective tissue is noticed. Furthermore, stratum intermedium, which plays a role in normal odontogenesis is absent, that explains the absence of enamel matrix.[1]
These observations have led to the consensus that ameloblastoma is probably the most primitive of odontogenic lesions, which does not show inductive interactions.[5] If it has, probably they end up as odontoameloblastomas.[5-7] Furthermore, some cases have been documented as dentinoameloblastomas, that exhibited features of ameloblastoma along with abundant eosinophilic homogeneous extracellular material, interpreted as dentinoid based on its intimate association with odontogenic epithelium and its morphologic, histochemical and ultrastructural characteristics, though odontoblast differentiation and typical enamel matrix were completely absent.[5,8,9] The present case also showed similar features with evidence of abundant eosinophilic, homogenous extracellular material simulating dentinoid.
These interesting tumors as in the present case suggest the possibility that aberrant neoplastic cells of pure epithelial origin are involved in the synthesis of dentinoid. This is contrary to currently accepted theory that only odontoblasts of ectomesenchymal origin are responsible for the formation of dentin. A study by Papagerakis et al. have given a plausible explanation for this phenomenon by clearly demonstrating that ameloblastic epithelial cells in mixed odontogenic tumors expressed gene products normally present in ectomesenchymal cells and resulted in conversion to co-express mesenchymal phenotype.[5,10] Thus, it is probable that neoplastic epithelial cells committed to ameloblastic differentiation can also produce the dentinoid in existence of some tumor-specific events.[5]
The pleomorphic cells observed in close proximity to the bony trabeculae in the peripheral areas of the stroma have been established in the present case as sarcomatous in nature based on their positivity for vimentin and negativity towards cytokeratin 19. Since this sarcomatous change was happening in the peripheral areas, this phenomenon seemed to adhere to the logical assumption that these areas being the most immature have the predilection to undergo such a change under the influence of some triggering factor.[5]
This sarcomatous change directed ones thought process to think in terms of ameloblastoma probably transforming into ameloblastic fibrosarcoma, however the supporting stroma observed in these areas was not ectomesenchymal in nature, which is a prerequisite to diagnose a lesion as ameloblastic fibrosarcoma, rather it was dense fibrous in nature, hence, preventing us to arrive at any concrete end point. Thus, one must ask the question as to whether too much importance is being given to the stromal features in diagnosing these odontogenic lesions? It is also possible to postulate that some of the well-formed mature collagenous fibres were the products of the differentiated fibroblasts before they transformed into malignant character.
The uniqueness of the granular cell change within this particular case was that this change was occurring in the stromal component as proved by its positivity for vimentin and negativity for cytokeratin, whereas in granular cell ameloblastoma the granular cell change is observed as an extensive transformation of the central stellate reticulum-like cells within the epithelial islands.[11] In granular cell variant of ameloblastic fibroma, the granular cell change is evident within the ectomesenchymal component,[12] but in the present case there was dense fibrous stromal component instead of an ectomesenchymal component. Hence, to the best of our knowledge this is the first case of ameloblastoma in which granular cell change was reported to be occurring within the stromal component.
Furthermore it is to be reported that the macroscopic observation, as shown in Figure 3, showed that within the solid component of the neoplasm there was central cystic change which contained another proliferating mass, thus giving a new dimension to think whether a new variant tumor was developing within the degenerated area.
The present case only makes us to believe that in longstanding ameloblastomas variable stromal changes also occur either as a defensive mechanism or vested nature of the body system resulting in bizarre behavior.
Source of Support
Nil.
Conflict of Interest
None declared.
References
- Maia Campos G. Ameloblastoma, a behavioral and histologic paradox (a philosophical approach). Braz Dent J 1990;1:5-15.
- Raubenheimer EJ, van Heerden WF, Noffke CE. Infrequent clinicopathological findings in 108 ameloblastomas. J Oral Pathol Med 1995;24:227-32.
- Reichart PA, Philipsen HP Introduction to Ameloblastomas. In: Odontogenic Tumors and Allied Lesions. London: Quintessence Publishing Co. Ltd.; 2004. p. 41-2.
- Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization Classification of Tumors. Pathology and Genetics of Head and Neck Tumors. Lyon: IARC Press; 2005. p. 283-328.
- Ide F, Horie N, Shimoyama T, Sakashita H, Kusama K So-called hybrid odontogenic tumors: Do they really exist? Oral Med Pathol 2001;6:13-21.
- Mosca RC, Marques MM, Barbosa SC, Marcucci M, Oliveira JX, Lascala CA. Odontoameloblastoma: Report of two cases. Indian J Dent Res 2009;20:230-4.
- Dive A, Khandekar S, Bodhade A, Dhobley A. Odontoameloblastoma. J Oral Maxillofac Pathol 2011;15:60-4.
- Slabbert H, Altini M, Crooks J, Uys P. Ameloblastoma with dentinoid induction: Dentinoameloblastoma. J Oral Pathol Med 1992;21:46-8.
- Matsumoto Y, Mizoue K, Seto K. Atypical plexiform ameloblastoma with dentinoid: Adenoid ameloblastoma with dentinoid. J Oral Pathol Med 2001;30:251-4.
- Papagerakis P, Peuchmaur M, Hotton D, Ferkdadji L,Delmas P, Sasaki S, et al. Aberrant gene expression in epithelial cells of mixed odontogenic tumors. J Dent Res 1999;78:20-30.
- Reichart PA, Philipsen HP. Odontogenic Tumors and Allied Lesions. London: Quintessence Publishing Co. Ltd.; 2004. p. 43-58.
- Reichart PA, Philipsen HP. Odontogenic Tumors and Allied Lesions. London: Quintessence Publishing Co. Ltd.; 2004. p. 121-8.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.