Thyrotoxicosis Associated Enhanced Systolic Function: Lessons on Possible Therapeutic Use of Thyroid Hormone in Selected Cases of Low Thyroid Hormone Levels and Poor Systolic Function
Citation: Anakwue RC. Thyrotoxicosis Associated Enhanced Systolic Function: Lessons on Possible Therapeutic Use of Thyroid Hormone in Selected Cases of Low Thyroid Hormone Levels and Poor Systolic Function. Ann Med Health Sci Res. 2018;8:285-291
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Objectives: The heart and blood vessels are sensitive to the effects of excess thyroid hormones (THs). Echocardiography is an important investigative modality in detecting cardiac involvement in disease states and so was employed in finding out if the hyperdynamic state in thyrotoxicosis was associated with enhanced left ventricular systolic function. Methodology: Consultants in main hospitals in Enugu were requested to send in adult patients with thyrotoxicosis to the out patients department of UNTH, Enugu. The patients were recruited consecutively and diagnosis confirmed following clinical assessment and thyroid function tests. Echocardiography was done to assess enhanced left ventricular systolic function (indicated by any of these parameters VEF >75%, FS >42%, CO > 7 l/min, CI >4.3 l/min/m2, MVCF >1.9 cir/sec, PASV >120 cm/sec. Results: Thyrotoxicosis patients were leaner, had no significant difference in blood pressure when compared with controls. The patients also had significantly enhanced left ventricular systolic function when their FS (43.9%-p<0.05), CO (7.15l/min-p<0.05), and AOVMAX (144 cm/sec-p<0.05) were compared with controls. Values of free T3 also correlated positively with systolic echocardiographic parameters. Discussion and Conclusion: The effect of TH on the heart has led to interest in the therapeutic use of TH on cardiac diseases. Thyroid hormone could be used as replacement therapy in cardiac diseases in which there is background low thyroid hormone and poor systolic function or heart failure.
Keywords
Thyrotoxicosis; Enhanced systolic function; Lessons on thyroid hormone replacement; Poor systolic function
Introduction
Thyrotoxicosis is a condition in which there is excess circulating free tri-iodothyronine (FT3) and/or free thyroxine (FT4). Most studies support the hypothesis that T3 is the final mediator and that T4 is a prohormone, primarily because of the universal presence of T3, but not T4 nuclear receptors, in tissues responsive to thyroid hormone. [1]
Thyroid hormone has profound effect on many systems and metabolic process but the heart is particularly sensitive to its effects. [2-5] T3 has both direct and indirect effects on heart function. The direct effect appears to be mediated by changes in protein synthesis which then regulate myocardial gene expression. Thyroid hormone upregulates the following genes: myosin heavy-chain alpha, [6] Ca2+ ATpase; [7] Na+k+ATpase; [8] beta adrenergic receptor; [9] glucose transporter (Glut-4); [10] Cardiac troponin I, [11,12] and atrial natriuretic protein [13] thereby increasing systolic contractile function. Thyroid hormone also down-regulates the following genes: myosin heavy-chain beta, phospholamban and glucose transporter (Glut-1) [10] causing reduced cardiac contraction.
The effect of thyroid hormone on the vascular system is remarkable. [14] The hyperynamic cardiovascular state associated with thyrotoxicosis results from thyroid hormone induced peripheral vasodilatation which leads to increased venous return giving rise to increased cardiac contractility and output. [14]
Echocardiography has been shown to be sensitive in detecting cardiac involvement in diseased states. Echocardiographic studies of the left ventricular systolic function in thyrotoxicosis in Africa are few. To the best of our knowledge only four of such echocardiographic studies have been done in Nigeria including one by us on the Igbos who lives in the eastern part of Nigeria. [15-18] Thyrotoxicosis is a hypermetabolic and hyperdynamic cardiovascular state and so this study was designed to find out if there is enhanced left ventricular systolic function associated with this endocrine disorder.
Methods
Study population and equipment
The study recruited patients with thyrotoxicosis seen consecutively over one year period in the medical outpatients of University of Nigeria Teaching Hospital. The fifty patients aged 15years or older and of both sexes included in the study were referred from the consultants in the main hospitals in Enugu city. All the study participants gave consent. The study was assessed and approved by the UNTH ethical committee in keeping with international regulations.
Data was collected on patients’ demographic characteristics, clinical state in keeping with thyrotoxicosis and blood collected for thyroid function tests. The study used kits from Syntron Bioresearch Inc USA for the thyroid function tests. The Syntron kits have the following intra assay coefficient of variation: free T3 (6.8%), TSH (4.3%), total T3 (4.4%), total T4 (7.2%). [18] Comparison between Syntron kit and Abbot Kit showed a correlation coefficient of 0.0986918. Electrocardiography was done with a Hewlett Packard Electrocardiographic machine. Two-dimensional and m-mode transthoracic echocardiographic examinations were performed in all subjects using SONOS 2000 Echocardiographic machine with 3.5 MHz transducer according to the recommendations of American Society of Echocardiography. [19]
Exclusion criteria included patients with hypertension preceding thyrotoxicosis, and patients with diabetes mellitus, anaemia, coronary artery diseases or exposed to illicit drugs, herbal or any cardiotoxic agents. Fifty age and sex matched controls with no clinical or biochemical evidence of thyrotoxicosis and co-morbidities were also studied. Exclusion criteria were as in the patient population
Definitions
Thyrotoxicosis was deemed to be diagnosed if there was serum – free iodothyronine > 4.2 pg/l and a concomitant suppressed thyroidstimulating hormone level <0.5 uu/ml.
Left ventricular enhanced systolic function was established by any of the following: left ventricular ejection fraction (LVEF) >75%, fractional shortening (FS) >42%, cardiac index (CI) >4.3 litre/min/m2, cardiac output (CO) > 7 liters/min, mean velocity of circumferential fibre shortening (mVCF) > 1.9 cir/sec, peak aortic systolic velocity (AOVMAX) >120 cm /sec.
Statistical analysis
Continuous variables were expressed as mean ± SEM. Statistical comparison was performed using Students t test. Calculations were done using SPSS software (version 11). p ≤ 0.05 was considered significant. Correlation was done were appropriate to find out if there were relationships between parameters.
Results
Demographic characteristics
The baseline demographic and clinical variables of the 50 consecutive patients diagnosed with thyrotoxicosis are presented in Table 1. There were no significant differences when the mean age (43.5 ± 15.2 years) and height (160.9 ± 7.0 cm) of the thyrotoxicosis patients were compared with the age and height of the controls (44.3 ± 14.2 years and 160.3 ± 15.9 cm) respectively. However, thyrotoxicosis patients had significantly lower weight (57 ± 5.2 kg vs. 67.5 ± 8.4 kg, p<0.01) and body mass index (22.26 ± 5.6 vs. 24.2 ± 4.3 kg/m2, p<0.05) when compared with the control [Table 1a].
|  (A) Anthropometric data and blood pressure of patients and controls | |||||
| Parameters | Thyrotoxicosis Patients: Mean (SD) | Control: Mean (SD) | ‘t’ Test Value | p-value | |
| Age | 44 (3.1) | 43.5 (5.2) |  0.85 | >0.05 | |
| Weight (kg) | 56.0 (5.5) | 65 (7.6) | -6.4 | <0.05* | |
| Height (meters) | 159.5 (6.8) | 160.8 (15.9) | 1.35 | >0.05 | |
| Body surface area (m2) | 1.2 (2.4) | 1.6 (0.17) | 1.85 | <0.5* | |
| Body mass Index (Kg|m2) | 22.04 (6.4) | 25.09 (5.4) | -1.87 | <0.05* | |
| Pulse (beats |min) | 102 (11.9) | 78 (5.2) | 8.57 | <0.01* | |
| Systolic Blood Pressure (mmHg) | 124.2 (12.5) | 122.9 (2.8) | 0.12 | >0.05 | |
| Diastolic Blood Pressure (mmHg) | 75 (11.4) | 79.1 (6.5) | 1.5 | >0.05 | |
|  (B) Frequencies of symptoms in thyrotoxicosis patients | |||||
| Symptoms | Patients (percentage) | ||||
| Palpitation | 30 (60) | ||||
| Enlarged thyroid gland | 26 (52) | ||||
| Weight loss | 24 (48) | ||||
| Heat intolerance | 23 (46) | ||||
| Tremulousness | 21 (42) | ||||
| Proptosis (Graves’ patients) | 20 (40) | ||||
| Increased sweating | 18 (38) | ||||
| Polyphagia | 16 (32) | ||||
Key: *represents significant value
Table 1: (A) Anthropometric data and blood pressure of patients and controls; (B) Frequencies of symptoms in thyrotoxicosis patients.
Clinical characteristics
The most common symptom found among thyrotoxicosis patients was palpitation. (in 30 patients-60%). Other symptoms and their occurrences in the thyrotoxicosis patients are shown in Table 1b.
Graves’ disease was the most common cause of thyrotoxicosis in this study (31 patients -62%). The mean heart rate was significantly higher in thyrotoxicosis patients: 104 bpm (range: 98-140 bpm) than in the control group 75.1 (range: 72-83 bpm), p<0.01. There was no significant difference between the systolic blood pressure and diastolic blood pressure of the thyrotoxicosis patients and the control groups.
Echocardiographic findings
Left ventricular systolic function parameters were compared between thyrotoxicosis patients and control and shown in Table 2. Thyrotoxicosis patients had higher values than the control for left ventricular ejection fraction, fractional shortening, cardiac output, cardiac index, peak aortic systolic velocity, and velocity of circumferential fibre shortening. The differences were found to be significant (all p< 0.01) and demonstrated the presence of left ventricular enhanced systolic function in thyrotoxicosis patients.
| Parameters | Mean values for Thyrotoxicosis Patients (SD) [reference range] | Mean values for Control (SD) | t-test value | p-value |
|---|---|---|---|---|
| LVEFR (%) | 69.7 (10.2) [41-79] | 59 (3.3) | 2.47 | <0.01* |
| FS (%) | 43.9 (9.8) [12-48] | 36.83 (8.4) | 2.8 | <0.01* |
| Cardiac output (litres) | 7.15 (1.47) [3.5-8.0] | 4.7 (0.67) | 7.64 | <0.01* |
| Cardiac Index l/min/m2 | 4.1 (0.84) [2.1-4.8] | 3.2 (0.29) | 4.13 | <0.01* |
| Peak aortic systolic velocity (cm/s) | 144 (26.7) [81-165] | 113 (11.5) | 7.5 | <0.01* |
| VCF (circumferences/second) | 1.74 (0.34) 1.8-2.1] | 1.21 (0.12) | 11.0 | <0.01* |
Key: LVEFR: Left Ventricular Ejection Fraction; FS: Fractional Shortening; VCF: Velocity of Circumferential Fiber Shortening
Table 2: Comparison of parameters of left ventricular systolic function between thyrotoxicosis patients and control.
A comparison of values of free T3 with systolic echocardiographic parameters [Table 3] showed positive correlation as against negative correlation with TSH indices. The degree of correlation was more strongly with LVEF, AOVMAX and MVCF.
| Parameters | Pearson’s Correlation Coefficient for TSH | p-value (TSH) | Pearson’s Correlation Coefficient for Free T3 | p-value (FT3) |
|---|---|---|---|---|
| LVEFR | -0.402 | <0.01 | 0.365 | <0.01 |
| FS | -0.396 | <0.01 | 0.347 | <0.01 |
| CO | -0.283 | <0.05 | 0.319 | <0.05 |
| CI | -0.259 | <0.05 | 0.301 | <0.05 |
| AOVMAX | -0.406 | <0.01 | 0.390 | <0.05 |
| MVCF | -0.517 | <0.01 | 0.370 | <0.01 |
| LVE | -0.368 | <0.01 | 0.301 | <0.01 |
| LVA | -0.309 | <0.01 | 0.307 | <0.01 |
| LVE/A | -0.013 | >0.05 | 0.133 | >0.05 |
| LVIVRT | -0.033 | >0.05 | 0.129 | >0.05 |
| LVDT | -0.064 | >0.05 | 0.206 | >0.05 |
| PFR | -0.068 | >0.05 | 0.267 | >0.05 |
| RVE | -0.402 | <0.01 | 0.417 | <0.01 |
| RVA | -0.396 | <0.01 | 0.365 | <0.01 |
| RVE/A | -0.145 | >0.05 | -0.145 | >0.05 |
| RVDT | -0.158 | >0.05 | -0.106 | >0.05 |
*p-value <0.05 is significant represents significant value
Table 3: Correlation of thyroid stimulating hormone and free T3 with echocardiographic parameters.
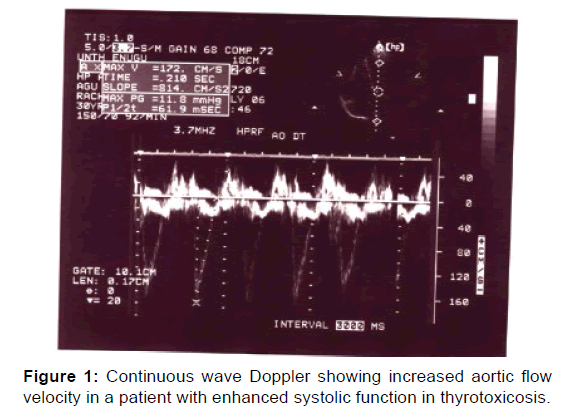
A continuous wave Doppler demonstrating increased aortic velocity in a patient with enhanced systolic function in thyrotoxicosis is shown in Figure 1.
Discussion
Cardiac output is increased 50 to 300% in thyrotoxicosis patients than in normal patients. [20] This form of enhanced systolic function was found in this study as demonstrated by increase in aortic maximal forward flow velocity in 11 patients (22%) [Figure 1]. Table 2 documented the significantly increased systolic findings in the subjects studied compared with control. And Table 3 showed that thyroid hormones correlated positively with systolic function parameters supporting the evidence for enhanced systolic function.
Enhanced systolic function is due to both a direct action of TH on the heart and an indirect action on the peripheral vessels. The former is due to the preferential expression of the alpha-myosin heavy chain which has greater ATPase activity and thus augments cardiac contraction as well as increased activity of calcium activated ATPase leading to increased inotropism. The latter is due to decreased systemic vascular resistance increased venous return and blood volume and cardiac contractility. [4]
The cellular mechanisms governing the entry of triiodothyronine (T3), the biologically active thyroid hormone, into the cardiomyocyte through specific transport proteins located within the cell membrane [21] has been defined. We now know that systole preceeds the entry of T3 and that T3 enters the nucleus and interacts with specific transcriptional activators (nuclear receptor alpha-1) or repressors (nuclear receptor alpha-2). The combined effect of bound T3 and recruited cofactors, leads the thyroid hormone-receptor complex to bind (nuclear receptor -alpha1) or release (nuclear receptor alpha-2) specific sequences of DNA (thyroid-responsive elements) that, in turn, by acting as cis- or trans-regulators, modify the rate of transcription of specific target genes. [22] Myosin heavy chains and the sarcoplasmic reticulum proteins involved in the regulation of intracellular calcium handling. [23-26]
Myocardial relaxation is controlled by the rate of calcium reuptake into the lumen of the sarcoplasmic reticulum during diastole which itself is modulated by sarcoplasmic reticulum calcium activated ATPase. [25,26] This explains why in diastole, the thyroid hormone upregulates expression of the sarcoplasmic reticulum calcium-activated ATPase and down-regulates expression of phospholamban, thereby enhancing myocardial relaxation. [25,26]
This study is the first report on enhanced systolic function in thyrotoxicosis patients in Nigeria and perhaps in sub-Saharan Africa. Friedman et al. [27] confirmed enhanced systolic function by measuring the echocardiographic tracing of the septum and left posterior wall. Kral et al. in Czecholoslavakia [28] studied 12 patients with hyperthyroidism and documented significant increase in mean VCF as well as cardiac index, stroke volume and left ventricular end diastolic volume. Our study showed an increase by 17% in the mean VCF of thyrotoxicosis patients when compared with the control (1.74 vs. 1.41 circumferences/second).
Marcisz et al. in Poland while studying the hyperthyroidism noted that it was associated with enhanced systolic function. [29] They demonstrated increased left ventricular ejection function, fractional shortening, mean VCF, cardiac index and output- pressure index. Additionally there was a significant shortening of pre- ejection period, left ventricular ejection time, pre-ejection period index and left ventricular ejection time index; all these are evidences of enhanced systolic function in thyrotoxicosis patients. These values are also comparable with that obtained in animal studies involving 103 cats with hyperthyroidism in which Bond et al. [30] showed enhancement of indices of contractility. Feldman et al. [31] had recorded strong positive correlation between the level of thyroid hormone and the change in contractile state represented by mean VCF.
Rationale of TH replacement therapy in heart diseases
The effect of TH on the heart has led to interest in the therapeutic use of TH on cardiac diseases. Thyroid hormone could be used as replacement therapy in cardiac diseases in which there is background low thyroid hormone and poor systolic function or heart failure. There are research findings which provide evidence that TH is important in modulating cardiac function physiologically and in disease states. There are also new data that show that mildly altered TH function is negatively correlated with risk factors and events in patients with cardiovascular diseases. [32]
We had earlier published a work on congestive heart failure in subjects with thyrotoxicosis in a black community and eluded to the fact that thyrotoxicosis could cause heart failure.18 The fact that we are proposing the use of thyroid hormone for treatment of heart failure in this construct may seem like a paradox. However, thyrotoxicosis induced heart failure may be due to autoimmune destruction of cardiac myocytes in addition to other haemodynamic factors like hypervolemic burden, tachyarrhythmias, congestive circulation secondary to excess sodium and fluid retention. [32,33]
Thyrotoxicosis induced heart failure has a different patho-clinical basis from the therapeutic use of thyroid hormone in heart failure. The former exposes the pathophysiological mechanism of excess thyroid hormones on the heart, and the latter exploits the therapeutic use of thyroid hormone replacement in cardiac conditions particularly if there is low thyroxine.
It is known that hyperthyroidism and hypothyroidism can cause cardiovascular disease. Both conditions can cause mild cardiovascular diseases which may progress to myocardial fibrosis and eventually heart failure. Hypothyroidism induced heart disease tend to regress with thyroid hormone replacement and the reverse is the case for thyrotoxicosis. [34-36]
The effect of thyroid hormone on cardiac structure and function has also been demonstrated in patients without overt clinical hypothyroidism. This group of patients are said to have subclinical hypothyroidism (scHypo). [37,38] There are clinical observations that suggest a strong link between scHypo and poor outcome in patients with and without heart disease. [39-45] Many reports now suggest that scHypo is a risk factor in heart disease. [39,42,46-49] Another report showed that in chronic heart failure patients, TSH levels even slightly above normal range are independently associated with a greater likelihood of HF progression. [50] There are also experimental data that support that scHypo may cause reversible left ventricular (LV) diastolic dysfunction in subjects with TSH >10 μIU/mL. [51]
Low levels of myocardial T3 are found not only in hypothyroid disease but also in nonthyroid conditions like heart failure, myocardial infarction and cardiomyopathy because of the hearts’ inability to generate T3 from precursor T4. Where Low circulating levels of T3 in the absence of primary thyroid hypofunction have been found in 20% to 30% of patients with dilated cardiomyopathy. [52,53]
The conclusion is that low TH level is associated in heart diseases particularly so in those cardiovascular states with significant injury to the myocytes with myocardial infarction being an outstanding example. [54] There is now evidence. We now know that mild TSH abnormalities play an important role in the myocardial response to acute ischemia [55,56] and that thyroid hormone levels correlate with the clinical stage of heart failure with lower levels found in patients with lower NYHA class. Also lower TH levels have been associated with post-operative periods in patients who had undergone cardiopulmonary or coronaryaortic bypass surgery. [57-60]
The role of thyroid hormone in the endogenous regeneration of damaged heart
There is evidence that treatments targeting the TH signaling may promote endogenous regeneration of the damaged myocardium. [61-68] As a result TH treatment early after infarction resulted in marked improvement of contractile indices independent of loading conditions, due to favorable cell remodeling as indicated by the switch of MHC isoform expression from the fetal to adult pattern. [64] TH also increased cardiac mass and normalized wall stress while it preserved left ventricular geometry to the ellipsoidal shape leading to enhanced myocardial performance as indicated by significant improvement of left ventricular ejection fraction. [65]
Thyroid replacement therapy in patients with heart disease
It was found that during hypothyroidism MHC alpha mRNA was markedly lower, the MHC beta was high and the phospholamban was 10-fold higher in the hypothyroid heart when compared with euthyroid heart. When euthyroidism was restored, all changes reverted to normal and a normal cardiac function was restored. [69] These observations are the basis of the so-called activation of a fetal gene program [70,71] secondary to the hypothyroid state. These contribute to the decreased contractile function typical of adult HF and a reversal of genotype/ phenotype to a more fetal-like cardiac state.
It has been demonstrated that the activation of the fetal gene program observed during evolution of heart failure in adult life which appears to be potentially reversible when the TH profile is normalized. The restoration of a physiological thyroid hormone/thyroid receptor interaction might counteract progression of heart disease by different mechanisms including: (1) a positive remodeling through the modulation of myocardial gene expression; (2) an improvement in cardiac systolic and diastolic function with a consequent improvement in hemodynamics; (3) an improvement in myocardial perfusion and (4) a positive effect on quality of life by combining multisystem actions of the TH. The rationale for systemic administration of TH is based on the additional systemic actions of THs, in particular on skeletal muscle, kidney, and brain that could be important components of the progression from organ-limited to whole-body disease.
Thyroid hormone replacement therapy aimed at optimizing TH signalling in the presence of heart failure can be achieved through the following: (1) administration of synthetic L-T4 (3) administration of a TH analogue, (4) genetic manipulation of the expression of cardiovascular deiodinases, to increase local production and bioavailability of T3; (5) genetic manipulation of the cardiovascular thyroid hormone receptor pattern to increase hormonal signalling at the receptor level while preserving T3 availability. Presently, the first three approaches have been applied in a few human studies, and with a low number of patients [72-74] whereas the remaining therapeutic options are still in the experimental animal stage. [75,76]
L-T4 Administration ensures that the main physiological pathway of secretion and peripheral metabolism of TH system is preserved. Moruzzi et al. published two studies in which synthetic L-T4 was given orally at the “physiological” dose of 0.1 mg per day and cardiovascular effects were assessed after short-term (one-week) treatment and after continuous three-month treatment. [77,78] These two studies showed that L-T4 was well tolerated and induced significant improvement in cardiac pump function, consisting of enhanced resting LVEF, resting cardiac output, and functional capacity during exercise.
The therapeutic use of L-T3 the physiological pattern of peripheral conversion from T4 into T3, which is impaired in presence of a low-T3 syndrome, is bypassed through direct application of the biologically active TH. In a study in which L-T3 was administered at a “physiological” dose of 20 μg/day/m2 body surface for a period of 96 h in six patients with advanced heart failure and low-T3 syndrome who were undergoing stable conventional treatment and salt intake. L-T3 constant infusion induced a progressive reduction in systemic vascular resistance and an increase in left ventricular ejection fraction and cardiac output, the latter invasively monitored by Swan-Ganz catheter; urinary output also improved, whereas no changes in heart rate and systemic arterial BP were observed. [79-81] Thyroid hormone analogue 3,5 has similar positive effects than the natural thyroid hormone but lower adverse effects. [82,83]
Conclusion
Thyroid hormone metabolic disorder has been identified as a risk factor for cardiovascular diseases. [43,52] Increasingly, the hypothyroid state, and in particular a low triiodothyronine level, has been associated with a reduced cardiac performance and poor prognosis in HF, even in the presence of normal thyroid-stimulating hormone levels. [43,44,52,53]
Studies which have demonstrated enhanced systolic function in thyrotoxicosis supports the hypothesis that thyroid hormone could be useful in the treatment of heart conditions in which poor systolic function and low thyroid hormone level are present. There is increasing evidence that thyroid hormones also produce remarkable improvements in cardiac remodeling, including beneficial changes in myocyte shape, microcirculation, and collagen. [84] There is need for more studies in large populations to help map out clearly the indications for thyroid replacement therapy in patients with and without cardiac disease. This is important particularly since there are presently very few new drug discoveries in the area of heart failure pharmacotherapy.
Conflict of Interest
The author discloses that he has no conflicts of interest.
REFERENCES
- Dillman WH. Biochemical basis of thyroid hormone action in the heart. Am J Med. 1990;88:626-627.
- Polikar R, Burger AG, Scherrer U, Nicod P. The thyroid and the heart. Circulation. 1993;1435-1437.
- Davis PJ, Davis FB. Acute cellular actions of thyroid hormone and myocardial function. Ann Thoracic Surg. 1993;56:S16-S17.
- Dillman WH. Cardiac function in thyroid disease: Clinical features and management considerations. Ann Thorac Surg. 1993;56:S9-S10.
- Rohrer D, Dillmann WH, Thyroid hormone markedly increases the mRNA coding for sarcoplasmic reticulum Ca2 -ATPase in the rat heart. J BioChem. 1988;263:6941-6944.
- Tsika RW, Bahl JJ, Leinwand LA, Morkin E. Thyroid hormone regulates expression of a transfected human alpha myosin heavy chain fusion gene in fetal rat heart cells. Proc Natl Acad Sci USA. 1990;87:379-381.
- Zarina-Herzberg A, Marques J, Sukovich D, Periasamy M. Thyroid hormone receptor modulates the expression of the rabbit cardiac sarcoendoplasmic reticulum Ca(2+)-ATPase gene. J Biol Chem.1994;269:1460-1461.
- Orlowski J, Lingrell JB. Thyroid and glucocorticoid hormones regulate the expression of multiple Na, K-ATPase genes in cultered neonatal rat cardiac myocyte. J Biol Chem. 1990;265:3462-3464.
- Bahouth SW. Thyroid hormones transcriptionally regulates the b-1 adrenergic receptor gene in cultured ventricular myocyte. J Biol Chem. 1991;266:15863-15864.
- Castelló A, Rodríguez-Manzaneque JC, Camps M, Pérez-Castillo A, Testar X, Palacín M, et al. Perinatal hypothyroidism impairs the normal transition of GLUT4 and GLUT1 glucose transporters from fetal to neonatal levels in heart and brown adipose tissue. Evidence for tissue-specific regulation of GLUT4 expression by thyroid hormone. J Biol Chem. 1994;269:5905-5906.
- Averyhart-Fullard V, Fraker LD, Murphy AM, Solaro RJ. Differential regulation of slow-skeletal and cardiac troponin I mRNA during development and by thyroid hormone in rat heart. J Mol Cell Cardiol. 1994;26:609-611.
- Dieckman LJ, Solaro RJ. Effect of thyroid status on thin-filament Ca2+ regulation and expression of troponin I in perinatal and adult rat hearts. Circ Res. 1990;67:344-346.
- Fullerton MJ, Stuchbury S, Krozowski ZS, Funder JW. Altered thyroidal status and the in vivo synthesis of atrial natriuretic peptide in the rat heart. Mol Cell Endocrinol. 1990;69:227-229.
- Park KW, Dai HB, Ojamaa K. The direct vasomotor effect of thyroid hormones on rat skeletal muscle resistance arteries. Anesthesia Annals. 1997;85:734-738.
- Kolawole BA, Balogun MO, Akinsola A. Thyrotoxicosis and heart-a review of the literature. Nigerian Journal of Medicine. 2001;16:50-54.
- Danbauchi SS, Anumah FE, Alhassan MA, Oyati A, Isah HS, Onyemelukwe GC, et al. Thyrocardiac Disease in Zaria: Clinical and Echocardiographic Characteristics. E-chocardiography Journal. 2004;2:1-5.
- Ogbera A, Isiba A. The scope of cardiac complications of thyrotoxicosis in Lagos, Nigeria. Endocrine Absracts. 2007;13:301.
- Anakwue RC, Onwubere BJC, Anisiuba BC, Ikeh VO, Mbah AU, Ike SO. Congestive heart failure in subjects with thyrotoxicosis in a black community Vascular Health and Risk Management. 2010;6:473-477.
- Klein I, Ojama K. Thyroid hormone and the cardiovascular system. The New England J of Med. 2001;344:501-509.
- Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: Results of a survey of echocardiuographic measurements. Circulation. 1978;58:1072.
- Everts ME, Verhoeven FA, Bezstarosti K, Moerings EP, Hennemann G, Visser TJ, et al. Uptake of thyroid hormone in neonatal rat cardiac myocytes. Endocrinology. 1996;137:4235-4242.
- Brent GA. The molecular basis of thyroid hormone action. N Engl J Med. 1994;331:847-853.
- Morkin E. Regulation of myosin heavy chain genes in the heart. Circulation. 1993;87:1451-1460.
- Ojamaa K, Klemperer JD, Klein I. Acute effects of thyroid hormone on vascular smooth muscle. Thyroid. 1996a;6:505-512.
- Dillmann WH. Biochemical basis of thyroid hormone action in the heart. Am J Med. 1990;88:626-630.
- Kiss E, Jakab G, Kranias EG, Edes I. Thyroid hormone induced alteration in phospholamban protein expression: regulatory effects on sarcoplasmic reticulum Ca2+ transport and myocardial relaxation. Circ Res. 1994;75:245-251.
- Friedman MJ, Okada RD, Ewy GA, Hellman DJ. Left ventricular systolic and diastolic function in hyperthyroidism. American Heart Journal. 1982;104:6:1303-1308.
- Kral J, Hradec J, Limanova J. Heart in thyroid diseases. Cor Vasa. 1992;34:108-114.
- Marcisz C, Kucharz EJ, Jonderko G, Wojewódka J. The Systolic function of the Left Ventricle of heart in patients with hyperthyroidism during therapy. Pol Arch Medicine Wewn. 2001;105:131-138.
- Bond BR, Fox PR, Peterson ME, Skavaril RV. Echocardiographic findings in 103 cats with hyperthyroidism, Journal of American Veterinarian Medical Association. 1988;192:1546-1549.
- Feldman T, Borrow KM, Sarne DH, Neumann A, Lang RM. Myocardial mechanics in hyperthyroidism: Importance of left ventricular loading conditions, heart rate and contractile state. Journal of American College of Cardiology. 1986;7:972.
- Gerdes AM, Iervasi G. Thyroid replacement therapy and heart failure. Circulation. 2010;122:385-393.
- Siu C, Yeung C, Lau C, Kung AWC, Tse H. Incidence, clinical characteristics and outcome of congestive heart failure as the initial presentation in patients with hyperthyroidism. Heart. 2007;93:483-487.
- Chen WJ, Lin KH, Lee YS. Molecular characterization of myocardial fibrosis during hypothyroidism: Evidence for negative regulation of the pro-alpha1(I) collagen gene expression by thyroid hormone receptor. Mol Cell Endocrinol. 2000;162:45-55.
- Yao J, Eghbali M. Decreased collagen gene expression and absence of fibrosis in thyroid hormone-induced myocardial hypertrophy: Response of cardiac fibroblasts to thyroid hormone in vitro. Circ Res. 1992;71:831-839.
- Tang YD, Kuzman JA, Said S, Anderson BE, Wang X, Gerdes AM. Low thyroid function leads to cardiac atrophy with chamber dilatation, impaired myocardial blood flow, loss of arterioles, and severe systolic dysfunction. Circulation. 2005;112:3122-3130.
- Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29:76-131.
- Surks MI, Ortiz E, Daniels GH, Sawin CT, Col NF, Cobin RH, et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA. 2004;291:228-238.
- Iervasi G, Molinaro S, Taddei MC, Galli E, Mariani F, et al. Association between increased mortality and mild thyroid dysfunction in cardiac patients. Arch Intern Med. 2007;167:1526-1532.
- Friberg L, Drvota V, Bjelak AH, Eggertsen G, Ahnve S. Association between increased levels of reverse triiodothyronine and mortality after acute myocardial infarction. Am J Med. 2001;111:699-703.
- Pingitore A, Landi P, Taddei MC, Ripoli A, L’Abbate A, Iervasi G. Triiodothyronine levels for risk stratification of patients with chronic heart failure. Am J Med. 2005;118:132-136.
- Iervasi G, Pingitore A, Landi P, Raciti M, Ripoli A, Scarlattini M. Low-T3 syndrome: A strong prognostic predictor of death in patients with heart disease. Circulation. 2003;107:708-713.
- Hamilton MA, Stevenson LW, Luu M, Walden JA. Altered thyroid hormone metabolism in advanced heart failure. J Am Coll Cardiol. 1990;16:91-95.
- Biondi B, Palmieri EA, Lombardi G, Fazio S. Effects of subclinical thyroid dysfunction on the heart. Ann Intern Med. 2002;137:904-914.
- Hak AE, Pols HA, Visser TJ, Drexhage HA, Hofman A, Witteman JC. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: The Rotterdam Study. Ann Intern Med. 2000;132:270-278.
- Kvetny J, Heldgaard PE, Bladbjerg EM, Gram J. Subclinical hypothyroidism is associated with a low-grade inflammation, increased triglyceride levels and predicts cardiovascular disease in males below 50 years. Clin Endocrinol (Oxf). 2004;61:232-238.
- Razvi S, Ingoe L, Keeka G, Oates C, McMillan C, Weaver JU. The beneficial effect of l-thyroxine on cardiovascular risk factors, endothelial function, and quality of life in sub-clinical hypothyroidism: Randomized, crossover trial. J Clin Endocrinol Metab. 2007;92:1715-1723.
- Asvold BO, Bjoro T, Nilsen TI, Gunnell D, Vatten LJ. Thyrotropin levels and risk of fatal coronary heart disease: the HUNT study. Arch Intern Med. 2008;168:855-860.
- Silva-Tinoco R, Castillo-Martinez L, Orea-Tejeda A, Orozco-Gutiérrez JJ, Vázquez-Díaz O, Montaño-Hernández P. Developing thyroid disorders is associated with poor prognosis factors in patient with stable chronic heart failure. Int J Cardiol. 2011;147:e24-e25.
- Iacoviello M, Guida P, Guastamacchia E, Triggiani V, Forleo C, Catanzaro R. Prognostic role of sub-clinical hypothyroidism in chronic heart failure outpatients. Curr Pharm Des. 2008;14:2686-2692.
- Vanderpump MP. How should we manage patients with mildly increased serum thyrotrophin concentrations? Clin Endocrinol (Oxf) 2009.
- Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344:501-509.
- Iervasi G, Molinaro S, Landi P, Taddei MC, Galli E, Mariani F. Association between increased mortality and mild thyroid dysfunction in cardiac patients. Arch Intern Med. 2007;167:1526-1532.
- Friberg L, Werner S, Eggertsen G, Ahnve S. Rapid down-regulation of thyroid hormones in acute myocardial infarction: Is it cardioprotective in patients with angina? Arch Intern Med. 2002;162:1388-1394.
- Asvold BO, Vatten LJ, Nilsen TI, Bjøro T. The association between TSH within the reference range and serum lipid concentrations in a population-based study: The HUNT Study. Eur J Endocrinol. 2007;156:181-186.
- Franklyn JA, Gammage MD, Ramsden DB, Sheppard MC. Thyroid status in patients after acute myocardial infarction. Clin Sci (Lond). 1984;67:585-590.
- Hamilton MA, Stevenson LW, Luu M, Walden JA. Altered thyroid hormone metabolism in advanced heart failure. J Am Coll Cardiol. 1990;16:91-95.
- Holland FW II, Brown PS Jr, Weintraub BD, Clark RE. Cardiopulmonary bypass and thyroid function: a "euthyroid sick syndrome." Ann Thorac Surg. 1991;52:46-50.
- Klemperer JD, Klein I, Gomez M, Helm RE, Ojamaa K, Thomas SJ, et al. Thyroid hormone treatment after coronary-artery bypass surgery. N Engl J Med. 1995;333:1522-1527.
- Bettendorf M, Schmidt KG, Tiefenbacher U, Grulich-Henn J, Heinrich UE, Schönberg DK. Transient secondary hypothyroidism in children after cardiac surgery. Pediatric research. 1997;41:375.
- Pantos C, Mourouzis I, Cokkinos DV. Thyroid hormone as a therapeutic option for treating ischaemic heart disease: From early reperfusion to late remodelling. Vascular Pharmacology. 2010;52:157-165.
- Henderson KK, Danzi S, Paul JT, Leya G, Klein I, Samarel AM. Physiological replacement of T3 improves left ventricular function in an animal model of myocardial infarction-induced congestive heart failure, Circulation: Heart Failure. 2009;2:243-252.
- Chen YF, Kobayashi S, Chen J, Redetzke RA, Said S, Liang Q, et al., Short term triiodo-L-thyronine treatment inhibits cardiac myocyte apoptosis in border area after myocardial infarction in rats. Journal of Molecular and Cellular Cardiology. 2008;44:180-187.
- Pantos C, Mourouzis I Markakis K Dimopoulos A, Xinaris C, Kokkinos AD, et al. Thyroid hormone attenuates cardiac remodeling and improves hemodynamics early after acute myocardial infarction in rats. European Journal of Cardio-thoracic Surgery. 2007;32:333-339.
- Pantos C, Mourouzis I, Markakis KN, Tsagoulis N, Panagiotou M, Cokkinos DV. Long-term thyroid hormone administration reshapes left ventricular chamber and improves cardiac function after myocardial infarction in rats. Basic Research in Cardiology 2008;103:308-318.
- Forini F, Lionetti V, Ardehali H, Pucci A, Cecchetti F, Ghanefar M, et al. Early long-term L-T3 replacement rescues mitochondria and prevents ischemic cardiac remodelling in rats. Journal of Cellular and Molecular Medicine. 2011;15:514-524.
- Kalofoutis C, Mourouzis I Galanopoulos G, Dimopoulos A, Perimenis P, Spanou D, et al. Thyroid hormone can favorably remodel the diabetic myocardium after acute myocardial infarction. Molecular and Cellular Biochemistry. 2010;345:161-169.
- Pantos C, Mourouzis I, Tsagoulis N, Markakis K, Georgios G, Nikolaos R, et al. Thyroid hormone at supra-physiological dose optimizes cardiac geometry and improves cardiac function in rats with old myocardial infarction. Acta physiologica Polonica. 2009;12:49.
- Ladenson PW, Sherman SI, Baughman KL, Ray PE, Feldman AM. Reversible alterations in myocardial gene expression in a young man with dilated cardiomyopathy and hypothyroidism. Proceedings of the National Academy of Sciences. 1992;89:5251-5255.
- Colucci WS. Molecular and cellular mechanisms of myocardial failure. The American Journal of Cardiology. 1997;80:15L-25L.
- Khalife WI, Tang YD, Kuzman JA, Thomas TA, Anderson BE, Said S, et al. Treatment of subclinical hypothyroidism reverses ischemia and prevents myocyte loss and progressive LV dysfunction in hamsters with dilated cardiomyopathy. American Journal of Physiology-Heart and Circulatory Physiology. 2005;289:H2409-H2415.
- Hamilton MA, Stevenson LW, Fonarow GC, Steimle A, Goldhaber JI, Child JS, et al. Safety and hemodynamic effects of intravenous triiodothyronine in advanced congestive heart failure. The American journal of cardiology. 1998;81:443-447.
- Moruzzi P, Doria E, Agostoni PG. Medium-term effectiveness of L-thyroxine treatment in idiopathic dilated cardiomyopathy. The American Journal of Medicine. 1996;101:461-467.
- Iervasi G, Emdin M, Colzani RM, Placidi S, Sabatino L, Scarlattini M, et al. Beneficial effects of long-term triiodothyronine (T3) infusion in patients with advanced heart failure and low T3 syndrome. InProceedings of the 2nd International Congress on Heart Disease—New Trends in Research, Diagnosis and Treatment. 2001;549-553.
- Trivieri MG, Oudit GY, Sah R, Kerfant BG, Sun H, Gramolini AO, et al. Cardiac-specific elevations in thyroid hormone enhances contractility and prevent pressure overload-induced cardiac dysfunction. Proceedings of the National Academy of Sciences. 2006;103:6043-6048.
- Belke DD, Gloss B, Swanson EA, Dillmann WH. Adeno-associated virus-mediated expression of thyroid hormone receptor isoforms-α1 and-β1 improves contractile function in pressure overload-induced cardiac hypertrophy. Endocrinology. 2007;148:2870-2877.
- Moruzzi P, Doria E, Agostoni PG. Medium-term effectiveness of L-thyroxine treatment in idiopathic dilated cardiomyopathy. The American Journal of Medicine. 1996;101:461-467.
- Moruzzi P, Doria E, Agostoni PG, Capacchione V, Sganzerla P. Usefulness of L-thyroxine to improve cardiac and exercise performance in idiopathic dilated cardiomyopathy. American Journal of Cardiology. 1994;73:374-378.
- Hamilton MA, Stevenson LW, Fonarow GC, Steimle A, Goldhaber JI, Child JS, et al. Safety and hemodynamic effects of intravenous triiodothyronine in advanced congestive heart failure. The American journal of cardiology. 1998;81:443-447.
- Moruzzi P Doria E, Agostoni PG. Medium-term effectiveness of L-thyroxine treatment in idiopathic dilated cardiomyopathy. American Journal of Medicine. 1996;101:461-467.
- Goldman S, McCarren M, Morkin E, Ladenson P, Edson R, Warren S, et al. DITPA, a thyroid hormone analog to treat heart failure: phase II trial VA cooperative study. Journal of Cardiac Failure. 2008;14:796.
- Pingitore A, Iervasi G, Gerdes MA. Letter by Pingitore et al. regarding article, "DITPA (3,5-diiodothyropropionic acid), a thyroid hormone analog to treat heart failure: phase II trial Veterans Affairs cooperative study. Circulation. 2010;121:240.
- Ito K, Kagaya Y, Shimokawa H. Thyroid hormone and chronically unloaded hearts. Vascul Pharmacol. 2010;52:138-141.
- Khait L, Birla RK. Effect of thyroid hormone on the contractility of self-organized heart muscle. In vitro Cell Dev Biol Anim. 2008;44:204-213.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.