Undiagnosed Psoriatic Arthritis among Psoriasis Patients: A Meta-analysis of its Prevalence
Alanoud Farraj Al Alshaikh1*, Doaa Izzeldin Elsheikh2, Sarah Eissa Alkhamisi3, Lujain Shaker BinLadin2, Marwa Rashad Gammash1, Mohamed Samir Gul3, Fahad Saud Alanazi4, Manal Mansoor BinJawad5, Abeer Fahad Almutairi6, Moath Khalid Albusair7 and Omar Osama Shahada8
1Medical Intern, King Abdulaziz University, Jeddah, Saudi Arabia
2General Practitioner, Renew Clinics, Jeddah, Saudi Arabia
3General Practitioner, King Abdullah Medical City, Jeddah, Saudi Arabia
4General Practitioner, Ministry of Health, Tabuk, Saudi Arabia
5Medical Intern, Lublin University, Dammam, Saudi Arabia
6Medical Student, Unaizah College of Medicine, AlQassim University, Unaizah, Saudi Arabia
7Medical Intern, Imam Muhammed Ibn Saud University, Riyadh, Saudi Arabia
8Medical Intern, Taibah University, Medina, Saudi Arabia
- *Corresponding Author:
- Al-Anoud Farraj Al Al-Shaikh
Intern, King Abdulaziz University, Jeddah, Saudi Arabia
E-mail: Dr.alanoud.f.s@gmail.com
Citation: Al Al-Shaikh AF, et al. Undiagnosed Psoriatic Arthritis among Psoriasis Patients: A Meta-analysis of its Prevalence. Ann Med Health Sci Res. 2020;10: 949-953.
Abstract
Background: Evaluation of the prevalence of PsA in patients with psoriasis has ranged broadly from 7% to 48%. This variability is related in part to the definition of the disorder, source of patients, and geographic variation. Aim: This work aims to determine the prevalence of undiagnosed Psoriatic Arthritis (PsA) among psoriasis patients. Materials and Methods: A systematic search was performed over different medical databases to identify Dermatology studies, which studied the prevalence of undiagnosed PsA among psoriasis patients. Using the meta-analysis process, either with fixed or random-effects models, we conducted a meta-analysis on overall PsA prevalence as a primary outcome, and on psoriasis severity in PsA patients as a secondary outcome, which was assessed via Psoriasis Area and Severity Index (PASI). Results: Ten studies were identified involving 97762 psoriasis patients. The metaanalysis process revealed that the estimated pooled prevalence of undiagnosed PsA among psoriasis patients was (13.76%) (95% CI 6.689 to 22.856). We also found a highly significant increase in mean PASI, in PsA patients compared to the remaining dermal psoriasis (p<0.0001). Conclusion: To conclude, the findings are consistent with a low prevalence of PsA among patients with psoriasis and confirm a high percentage of undiagnosed cases with active arthritis among PsA patients in dermatologist’s office. Dermatologists should screen for PsA in their patients, especially those with risk characteristics and early signs.
Keywords
Undiagnosed psoriatic arthritis; Prevalence
Introduction
Psoriasis is a chronic skin disorder, affecting 2-3% of the population worldwide, which may be associated with psoriatic arthritis (PsA). PsA is a chronic inflammation of the musculoskeletal system that can be associated with inflammation in different organs which include the heart, eyes, aorta, lung, and kidney. PsA belongs to the group of spondyloarthritis, characterized by the usually negative rheumatoid factor test. Numerous sets of criteria had been developed by various groups of rheumatologists to diagnose PsA. [1] Evaluation of the prevalence of PsA in patients with psoriasis has ranged broadly from 7% to 48%. This variability is related in part to the definition of the disorder, source of patients, and geographic variation. The skin manifestations of psoriasis may additionally develop as long as a decade earlier than musculoskeletal manifestations, although joint disease might also arise at any time in the course of psoriasis. Increasing awareness by dermatologists about potential PsA in patients with psoriasis may be essential to prompt detection and therapeutic intervention and ultimately to inhibition of sickness progression, disability, and comorbidities. [2]
Similar, to patients with rheumatoid arthritis, early diagnosis, and the institution of disorder modifying antirheumatic drug treatment had been proven to affect long-time period morbidity. The delay within the diagnosis of PsA but remains a significant contributor to poor affected person outcomes. The early identification of patients with PsA amongst patients with psoriasis, therefore, assumes considerable significance. Patients with psoriasis are usually controlled by general practitioners or by dermatologists who can both rely on self-reported joint signs and symptoms to identify PsA or may be more proactive in elucidating the appropriate musculoskeletal (MSK) signs and symptom. [3]
The current proliferation of new treatment plans to treat psoriasis makes a critical assessment of efficacy paramount to prioritize therapeutic potential and reduce the patient hazard. Numerous studies have sought to objectively evaluate the numerous available psoriasis severity tools; however, no best instrument has yet been diagnosed. They reviewed all medical studies grading the severity of psoriasis for 29 years and analyzed assemble validity, content material validity, internal consistency, intraobserver variation, sensitivity to change, and acceptability/time required to perform the measurement. They observed that not one of the scoring tools met all the validation criteria, but that the Psoriasis Area and Severity Index (PASI) was the most extensively studied and most thoroughly validated. In the end, this study recommended the PASI for both clinical and scientific scoring of psoriasis severity. [4] This work aims to determine the prevalence of undiagnosed Psoriatic Arthritis (PsA) among psoriasis patients.
Literature Review
Our review came following the (PRISMA) statement guidelines. [5]
Study eligibility
The included studies should be in English, a journal published article, and a human study describing psoriasis patients. The excluded studies were non-English or animal studies.
Study identification
Basic searching was done over the PubMed, Cochrane library, and Google scholar using the following keywords: Undiagnosed Psoriatic Arthritis, Prevalence.
Data extraction and synthesis
Cohort, prevalence, and epidemiologic studies, which studied the psoriasis patients, will be reviewed. Outcome measures included overall PsA prevalence (as a primary outcome), and psoriasis severity in PsA patients as a secondary outcome, which was assessed via Psoriasis Area and Severity Index (PASI), (as a secondary outcome).
Study selection
We found 270 records, 220 excluded based on title and abstract review; 50 articles are searched for eligibility by full-text review; 18 articles cannot be accessed; 12 studies were reviews and case reports; 10 were not describing the functional outcome, leaving 10 studies that met all inclusion criteria.
Statistical methodology
The pooling of data, Proportions (%), standard mean differences (SMDs), with 95% confidence intervals (CI) were done, using MedCalc ver. 18.11.3 (MedCalc, Belgium). According to heterogeneity across trials using the I2-statistics; a fixed-effects model or random-effects model were used in the meta-analysis process.
Results
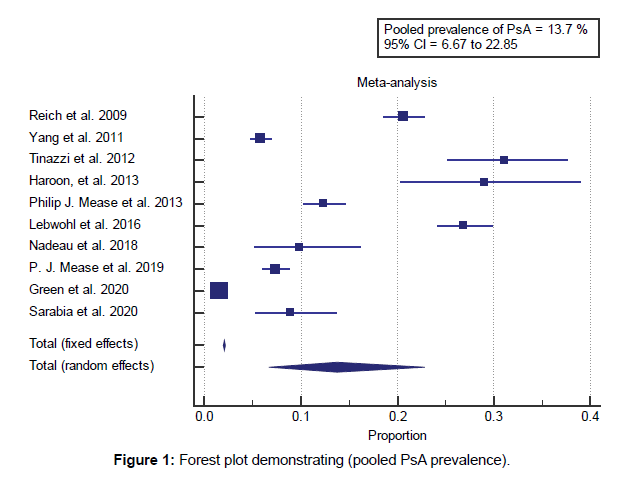
The included studies published between 2009 and 2020. Regarding the region of our studies, there were 6 European studies, 4 American studies, and 1 Asian study [Table 1]. [6-13] Regarding patients’ characteristics, the total number of patients in all the included studies was 97762 patients, with 2463 patients diagnosed as PsA [Table 1].
| Table 1: Patients and study characteristics. | |||||
| N | Author | Region | Number of patients | Age (Average years) | |
| Total psoriasis patients | PsA patients | ||||
| 1 | Reich et al. [6] | Europe | 1511 | 312 | 50 |
| 2 | Yang et al. [7] | Asia | 1928 | 112 | 39.2 |
| 3 | Tinazzi et al. [8] | Europe | 228 | 71 | 50 |
| 4 | Haroon, et al. [9] | Europe | 100 | 29 | 52 |
| 5 | Philip J. Mease et al. [10] | Europe/America | 949 | 117 | 51 |
| 6 | Lebwohl et al. [11] | America | 1005 | 270 | 57 |
| 7 | Nadeau et al. [12] | Europe | 133 | 13 | 52.8 |
| 8 | P. J. Mease et al. [13] | America | 1516 | 112 | 51 |
| 9 | Green et al. [14] | Europe | 90189 | 1409 | 48.5 |
| 10 | Sarabia et al. [15] | America | 203 | 18 | 52 |
The mean age of all patients was (50.3 years) [Table 1]. A meta-analysis study was done on 10 studies that described the prevalence of undiagnosed PsA; with an overall number of patients (N=97762) [Table 2]. [6-13] Each outcome was measured by:
| Table 2: Summary of outcome measures in all studies. | |||||||
| N | Author | Primary outcome | Secondary outcome | ||||
| PsA prevalence | Psoriasis severity (PASI) | ||||||
| Psoriasis patients | Prevalence (%) | PASI (psoriasis patients) | SD | PASI (PsA patients) | SD | ||
| 1 | Reich et al. [6] | 1511 | 20.6 | 11.5 | 3.4 | 14.3 | 3.8 |
| 2 | Yang et al. [7] | 1928 | 5.8 | 6 | 5.6 | 9.7 | 10.4 |
| 3 | Tinazzi et al. [8] | 228 | 31.1 | 5.8 | 2.88 | 6.8 | 3.18 |
| 4 | Haroon, et al. [9] | 100 | 29 | 1.89 | 1.14 | 2.4 | 1.13 |
| 5 | Philip J. Mease et al. [10] | 949 | 12.3 | 5.8 | 5.7 | 6.7 | 7 |
| 6 | Lebwohl et al. [11] | 1005 | 26.8 | - | - | - | - |
| 7 | Nadeau et al. [12] | 133 | 9.77 | - | - | - | - |
| 8 | P. J. Mease et al. [13] | 1516 | 7.4 | - | - | - | - |
| 9 | Green et al. [14] | 90189 | 1.5 | - | - | - | - |
| 10 | Sarabia et al. [15] | 203 | 806 | 2.4 | 3.8 | 5.8 | 10.9 |
a. Pooled Prevalence (Proportion)
• Of overall PsA prevalence.
b. Standard Mean Difference (SMD)
• For psoriasis severity (PASI), between psoriasis patients and PsA patients.
Concerning the primary outcome measure, we found 10 studies reported the prevalence of PsA, with a total number of patients (N=97762). I2 (Inconsistency) was 99.5% with a highly significant Q test for heterogeneity (p<0.0001), so the randomeffects model was chosen to assess the pooled prevalence. Using the random-effects model, the meta-analysis process revealed that, the estimated pooled prevalence of PsA among psoriasis patients was (13.76%) (95% CI 6.689 to 22.856) [Figure 1].
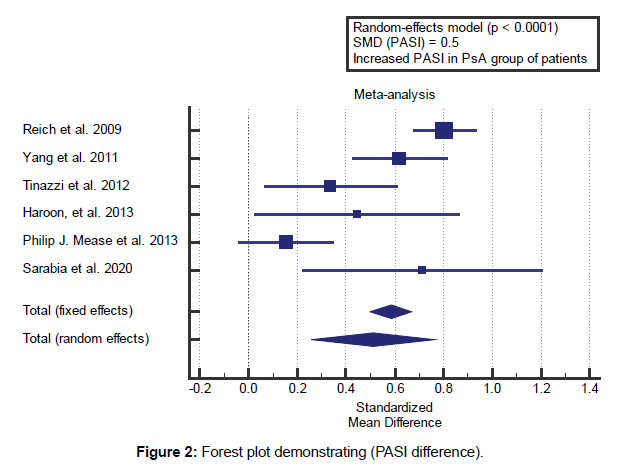
Concerning the secondary outcome measures, we found 6 studies reported PASI with a total number of patients (N=5578). I2 (Inconsistency) was 86% with highly significant Q test for heterogeneity (p<0.0001), so random-effects model was carried out; with overall SMD=0.5 (95% CI 0.257 to 0.761). Using the random-effects model, the meta-analysis process revealed a highly significant increase in mean PASI, in PsA patients compared to the remaining dermal psoriasis (p<0.0001) [Figure 2].
Discussion
This work aims to determine the prevalence of undiagnosed Psoriatic Arthritis (PsA) among psoriasis patients.
The included studies published between 2009 and 2020. Regarding the region of our studies, there were 6 European studies, 4 American studies, and 1 Asian study. Regarding patients’ characteristics, the total number of patients in all the included studies was 97762 patients, with 2463 patients diagnosed as PsA. The mean age of all patients was (50.3 years). Concerning the primary outcome measure, we found 10 studies reported the prevalence of PsA, with a total number of patients (N=97762). Using random-effects model, the metaanalysis process revealed that, the estimated pooled prevalence of PsA among psoriasis patients was (13.76%) (95% CI 6.689 to 22.856), which came in agreement with Villani et al. [14] Prey et al. [1] Camisa, [15] Yang et al. [7] Haroon, Kirby, and FitzGerald [3] and Ibrahim, Waxman, and Helliwell. [16] Villani et al. reported that their systematic search strategy leads to the final selection of 12 studies 7 epidemiological studies aimed at determining the prevalence of PsA in patients with skin psoriasis and 5 studies aimed at validating a PsA questionnaire. The prevalence of undiagnosed PsA was 15.5% when all studies had been considered and 10.1% whilst only epidemiological studies had been considered. [16] Prey et al. 2010 reported that the initial literature search diagnosed 2171 references.
Based totally on abstract reading and exclusion of studies not written in English or French, and without the manage group, the final selection included 20 epidemiological studies. With the eight studies using rheumatologically validated criteria, the prevalence of PsA among psoriasis patients spanned a wide range from 7% to 26%. [1] Camisa reported that, although PsA as a specific clinical entity remains questioned, it differs from rheumatoid arthritis (RA) in radiographic appearance, clinical presentation, and genetic predisposition. Although PSA and RA share some HLA allotypes, such as DR4, others, which include B27, BW38, DR7, and CW6, occur with significantly higher frequency in PSA. While HLA-Cw0602 has one of the most powerful most important histocompatibility complex (MHC) associations with psoriasis and psoriatic arthritis, it is observed in only 17% of patients with psoriasis versus 9% of controls. [15] Yang et al. reported that, among 1928 patients with psoriasis, 112 patients (5.8%) had PsA, of which 92% turned into newly identified. Oligoarthritis (48.2%) was the most common manifestation pattern, followed by spondylitis (26.8%), polyarthritis (19.6%), and classic distal interphalangeal (DIP) arthritis (5.4%). Enteritis was present in 26.8% and dactylitis in 13.4% of the patients. PsA had more severe skin disorders if compared with patients without PsA (mean PASI 9.7 vs. 6). [7]
clinical signs and symptoms of psoriasis on interview and examination. Extrapolating from the data of those people tested, the estimated (corrected) incidence was 13.8%. [16] Concerning the secondary outcome measures, we found 6 studies reported PASI with a total number of patients (N=5578). Using randomeffects model, the meta-analysis process revealed a highly significant increase in mean PASI, in PsA patients compared to the remaining dermal psoriasis (p<0.001), which came in agreement with Mattei, Corey, and Kimball, [17] Robinson, Kardos, and Kimball, [4] Schmitt and Wozel, [18] Villani et al. [14] and Mease. [19] Mattei, Corey, and Kimball reported that the tested studies stated PASI and DLQI data at baseline and 10- 16 weeks following treatment, when average percent reduction in PASI was plotted against the average decrease in DLQI (Dermatology life quality index score) throughout 22 treatment arms, a correlation coefficient value of 0.89 was observed (p<0.01). [17] Robinson, Kardos, and Kimball in 2012 reported that different trials in moderate to severe psoriasis had been reviewed. They recorded the percentage of patients achieving both 75% decrease in PASI rating (PASI 75) and PGA 0 or 1 (clear or almost clear) at eight to sixteen weeks, 17 to 24 weeks, and greater than 24 weeks of treatment with the investigational drug. Their literature review yielded 30 trials for the usage of biologic agents in moderate to extreme psoriasis. They observed that the 2 evaluation tools correlate very tightly except at the lower bounds of therapeutic efficacy. The r2 values for the correlation among PASI 75 and a score of clear or almost clear at the PGA have been 0.91 at 8 to 16 weeks and 0.89 at 17 to 24 weeks. [4]
Schmitt and Wozel reported that the Psoriasis Area and Severity Index (PASI) were developed to evaluate the effect of retinoid treatment in chronic plaque-type psoriasis. For different medical manifestations of psoriasis, the PASI is not adequate. Both depth and extent (BSA) of the psoriatic plaques are calculated separately for 4 anatomical regions (head, trunk, upper and lower extremities) by way of the medical doctor. The depth of erythema, desquamation, and induration is rated on a 5-point scale with zero indicating no involvement, 1 mild, 2 moderate, 3 extreme, and 4 very severe characteristics. The percentage of involvement of the four anatomical regions is assigned a numerical value of 0-6 with 0 indicating no involvement, 1=1- 9%, 2=10-29%, 3=30-forty nine%, 4=50-69%, 5=70-89% and 6=90-100% BSA involvement. They provide reasons why PASI>12 defines severe, PASI 7-12 mild and PASI<7 slight chronic plaque-type psoriasis. [18] Villani et al. reported that to check the effect of severity of the skin disorder, they restricted the analysis to studies that included patients with mean Psoriasis area Severity Index (PASI) scores 10 (prevalence, 18.5%) or to studies that included patients who were seen in a health center placing. [14] Mease reported that PASI measures both surface area and lesional severity of psoriasis. The head, upper extremities, lower extremities, and trunk are assessed separately and then combined the usage of weighting primarily based on the surface area represented through every area (head=0.1, upper extremities=0.2, trunk=0.3, and lower extremities=0.4). The degree of erythema, induration, and scale in each region is judged on a 0-4 scale, the sum of which represents disorder severity. The region of involvement of each vicinity is graded from 0-6, depending on the estimated percentage of lesional area (0=0%, 1=10%, 2=10-29%, 3=30-49%, 4=50-69%, 5=70-89%, and 6=90-100%). Within every area, the severity of psoriasis is estimated through erythema, induration, and desquamation. Severity parameters are measured on a scale of 0-4, from absent to very severe. there is poor sensitivity to change and responsiveness in slight psoriasis, so application is restricted to those with=3% BSA, and since it is uncommon to encounter an affected person with a PASI rating=40, nearly onehalf of the range of the scale is unused. [19] In a recent review of Rida, M.A. and Chandran, who stated that early diagnosis achieved by screening patients with a high risk of PsA e.g. those with a positive family history or skin disease. This is followed by careful examination and comprehensive evaluation for extra-musculoskeletal symptoms as well as musculoskeletal ultrasound to reach a final diagnosis and start early treatment. [20]
Conclusion
To conclude, the findings are consistent with a low prevalence of PsA among patients with psoriasis and confirm a high percentage of undiagnosed cases with active arthritis among PsA patients in the dermatologist’s office. Dermatologists should screen for PsA in their patients, especially those with risk characteristics and early signs
Competing Interests
The authors declare that they have no competing interests. All the listed authors contributed significantly to the conception and design of study, acquisition, analysis, and interpretation of data and drafting of the manuscript, to justify authorship.
References
- Prey S, Paul C, Bronsard V, Puzenat E, Gourraud PA, Aractingi S, et al. Assessment of risk of psoriatic arthritis in patients with plaque psoriasis: A systematic review of the literature. J Eur Acad Dermatol Venereol. 2010;24:31-35.
- Mease PJ, Gladman DD, Papp KA, Khraishi MM, Thaçi D, Behrens F, et al. Prevalence of rheumatologist-diagnosed psoriatic arthritis in patients with psoriasis in European/North American dermatology clinics. J Am Acad Dermatol. 2013;69:729-735.
- Haroon M, Kirby B, FitzGerald O. High prevalence of psoriatic arthritis in patients with severe psoriasis with suboptimal performance of screening questionnaires. Ann Rheum Dis. 2013;72:736-740.
- Robinson A, Kardos M, Kimball AB. Physician Global Assessment (PGA) and Psoriasis Area and Severity Index (PASI): Why do both? A systematic analysis of randomized controlled trials of biologic agents for moderate to severe plaque psoriasis. J Am Acad Dermatol. 2012;66:369-375.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: An explanation and elaboration. J Clin Epidemiol. 2009;62:e1-e34.
- Reich K, Krüger K, Mössner R, Augustin M. Epidemiology and clinical pattern of psoriatic arthritis in Germany: A prospective interdisciplinary epidemiological study of 1511 patients with plaque-type psoriasis. Br J Dermatol. 2009;160:1040-1047.
- Yang Q, Qu L, Tian H, Hu Y, Peng J, Yu X, et al. Prevalence and characteristics of psoriatic arthritis in Chinese patients with psoriasis. J Eur Acad Dermatol Venereol. 2011;25:1409-1414.
- Tinazzi I, Adami S, Zanolin EM, Caimmi C, Confente S, Girolomoni G, et al. The early psoriatic arthritis screening questionnaire: A simple and fast method for the identification of arthritis in patients with psoriasis. Rheumatology. 2012;51:2058-2063.
- Lebwohl MG, Kavanaugh A, Armstrong AW, Van Voorhees AS. US perspectives in the management of psoriasis and psoriatic arthritis: Patient and physician results from the population-based multinational assessment of psoriasis and psoriatic arthritis (MAPP) survey. Am J Clin Dermatol. 2016;17:87-97.
- Nadeau K, Briggs F, O’Neill S, Sumpton D, Cains G, Woods J, et al. THU0303 the early psoriatic arthritis screening questionnaire identifies patients with psoriatic arthritis amongst treated patients with psoriasis. BMJ Publishing Group Ltd. 2018.
- Mease PJ, Palmer JB, Hur P, Strober BE, Lebwohl M, Karki C, et al. Utilization of the validated Psoriasis Epidemiology Screening Tool to identify signs and symptoms of psoriatic arthritis among those with psoriasis: A cross-sectional analysis from the US-based Corrona Psoriasis Registry. J Eur Acad Dermatol Venereol. 2019;33:886-892.
- Green A, Shaddick G, Charlton R, Snowball J, Nightingale A, Smith C, et al. Modifiable risk factors and the development of psoriatic arthritis in people with psoriasis. Br J Dermatol. 2020;182:714-720.
- Sarabia S, Farrer C, Yeung J, Jerome D, Lee KA, Cook R, et al. The pattern of musculoskeletal complaints in patients with suspected psoriatic arthritis and their correlation with physical examination and ultrasound. J Rheumatol. 2020.
- Villani AP, Rouzaud M, Sevrain M, Barnetche T, Paul C, Richard MA, et al. Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: A systematic review and meta-analysis. J Am Acad Dermatol. 2015;73:242-248.
- Camisa C. Handbook of psoriasis. John Wiley & Sons, NY, USA. 2008.
- Ibrahim G, Waxman R, Helliwell PS. The prevalence of psoriatic arthritis in people with psoriasis. Arthritis Care Res. 2009;61:1373-1378.
- Mattei PL, Corey KC, Kimball AB. Psoriasis Area Severity Index (PASI) and the Dermatology Life Quality Index (DLQI): The correlation between disease severity and psychological burden in patients treated with biological therapies. J Eur Acad Dermatol Venereol. 2014;28:333-337.
- Schmitt J, Wozel G. The psoriasis area and severity index is the adequate criterion to define severity in chronic plaque-type psoriasis. Dermatology. 2005;210:194-199.
- Mease PJ. Measures of psoriatic arthritis: Tender and Swollen Joint Assessment, Psoriasis Area and Severity Index (PASI), Nail Psoriasis Severity Index (NAPSI), Modified Nail Psoriasis Severity Index (mNAPSI), Mander/Newcastle Enthesitis Index (MEI), Leeds Enthesitis Index (LEI), Spondyloarthritis Research Consortium of Canada (SPARCC), Maastricht Ankylosing Spondylitis Enthesis Score (MASES), Leeds Dactylitis Index (LDI), Patient Global for Psoriatic Arthritis, Dermatology Life Quality Index (DLQI), Psoriatic Arthritis Quality of Life (PsAQOL), Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-F), Psoriatic Arthritis Response Criteria (PsARC), Psoriatic Arthritis Joint Activity Index (PsAJAI), Disease Activity in Psoriatic Arthritis (DAPSA), and Composite Psoriatic Disease Activity Index (CPDAI). Arthritis Care Res. 2011;63:S64-S85.
- Rida MA, Chandran V. Challenges in the clinical diagnosis of psoriatic arthritis. Clin Immunol. 2020:108390.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.