Validation of Leiden Score in Predicting Progression of Rheumatoid Arthritis in Undifferentiated Arthritis in Indian Population
- *Corresponding Author:
- Dr. Ghosh K
Departments of Medicines and 2Anesthesiology, Murshidabad Medical College, Murshidabad
Flat 3B, Padma Apartment, 110 Dr. M N Saha Road, Kolkata-700 074, West Bengal, India.
E-mail:drkaushikghosh@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
CitationGhosh K, Chatterjee A, Ghosh S, Chakraborty S, Chattopadhyay P, Bhattacharya A, et al. Validation of leiden score in predicting progression of rheumatoid arthritis in undifferentiated arthritis in Indian population. Ann Med Health Sci Res 2016;6:205-10.
Abstract
Background: Leiden Score, is a very useful tool for predicting future development of rheumatoid arthritis (RA), among undifferentiated arthritis (UA) patients. This score has been validated in various western studies but rarely among south east Asian patients. Aims: To validate the Leiden early arthritis prediction rule in an Indian cohort of patients for predicting rheumatoid arthritis (RA) in undifferentiated arthritis (UA) patients and to formulate any simpler version of prediction score taking only clinical variables of original Leiden prediction rule. Subjects and Methods: In a group comparative longitudinal study model, 58 patients with early symmetrical polyarthritis were enrolled and baseline evaluation was done according to Leiden prediction rule and then 3 monthly. After 1 year, Leiden prediction score and chance of evolving into RA were calculated. Patients were divided into two groups: Those who developed RA and who did not. They were selected on random sampling process. Tender joint count (TJC), duration of morning stiffness, and duration of arthritis were selected as clinical variables for linear discriminant analysis with disease outcome being the dependent variable. Discriminant scores (D) for each patient was calculated. A receiver operating characteristic (ROC) curve was constructed with the discriminant score and compared with Leiden prediction score. Results: About 54% (27/50) of patients were diagnosed with RA and 46% (23/50) developed other rheumatologic condition or viral inflammatory arthritis or remained undifferentiated or attained complete remission. None of the patients with UA, who scored the regression coefficients 4 or less progressed to RA, and those who scored 7 or more, almost certainly progressed to RA. Unstandardized canonical discriminant coefficients for TJC (T), duration of morning stiffness (M), and duration of arthritis (A) were calculated. ROC curve was plotted with the formula: D = 0.164 × T + 0.066 × M + 0.012 × A − 2.838. Area under curve (AUC) at 95% confidence interval for our discriminant function was 0.845 (standard error [SE] 0.054). In comparison, AUC of Leiden prediction score was 0.897 (SE 0.043). Conclusions: Leiden prediction rule is highly applicable to UA patients to predict progression of RA in Indian patients and larger multi‑center study with larger cohorts is needed to validate the formulation we derived to predict RA.
Keywords
Early arthritis, Leiden prediction rule, Rheumatoid arthritis, Undifferentiated arthritis, Validation of prediction rule
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disorder that predominantly affects the small joints of the hands (and feet) symmetrically and causes significant deformity and disability. Early diagnosis of RA leads to better prevention of joint erosion and resulting deformity.[1] Studies have shown that RA can be predicted with reasonable accuracy with clinical and laboratory variables.[2] However, prediction models depend on the selected cohort of patients with undifferentiated arthritis (UA). This study aims to identify the clinical and laboratory variables that can predict RA and to formulate a prediction rule.
UA or e causa ignota is defined as any arthritis of recent onset (usually within 2 years of onset but varies widely) that poses the potential for a persistent path, without fulfilling the rheumatological classification criteria for specific conditions.
A significant proportion of patients who present with UA develop RA on follow-up.[3] The first 2 years after the onset of RA is considered as early RA. Maximum joint damage and bone erosion occur during this phase of the disease. However, in absence of typical clinical features, treatment with disease modifying agents is not possible. Therefore, the opportunity to halt or reverse the joint destruction is lost.
The clinical disease of RA is preceded by presence of serological markers such as rheumatoid factor (RF)[4] and anti-cyclic citrullinated polypeptide (CCP) antibody[5] almost by a decade. Therefore, there is an emphasis on ways to formulate a simple clinical prediction model to predict which patients of undifferentiated inflammatory polyarthritis will progress to full-blown RA at a very early stage.
Leiden Early Arthritis Clinic in Netherland derived a prediction rule for identifying RA amongst UA patients. It has been shown to have high specificity and sensitivity in European population studies.[3] However, this score is yet to be validated in Indian patients. The American College of Rheumatology (ACR) classification criteria, 1987, is applicable to established RA.[6] Its ability to diagnose early RA is, however, unsatisfactory.[7] Recently, European Union League Against Rheumatism (EULAR) has developed a new classification criteria for RA.[8] However, in Indian population, a large number of UA cases are due to postinfectious arthritis. In this study, we have compared the outcome of early arthritis in evolving RA according to EULAR criteria and also provided a new rule for predicting RA, which performs comparably to the Leiden score.
This study was undertaken to assess the validation of this prediction score in independent cohorts of patients with recent onset UA in our population who will progress to RA.
Subjects and Methods
Patient selection
Sample size was calculated with basis of ROC: 0.89 and expected error: 0.05. Assuming equal numbers participants in both groups. Sample size in each group was calculated 24. With view of drop-out rate, 5 number of sample were taken additionaly in each group.With a group comparative longitudinal study model, patients from both rural and urban catchment areas of Nilratan Sircar Medical College and Hospital, Kolkata, who attended Rheumatology Clinic in outpatients and inpatient departments and satisfied the following selection criteria, were evaluated over a period of 1 year in this study from March 2011 to March 2012.
By using the inclusion and exclusion criteria, a total 56 patients were enrolled in this study. They were entered in different groups by random sampling.
Ethical clearance
Ethical clearance was obtained from the Institutional Ethical Board, Nilratan Sircar Medical College and Hospital, Kolkata. Before the collection of data, all the patients were informed about the study objective, name of the researcher, risk and benefit of participation in the study. A written informed consent was taken from each and every participant of both groups. They were also allowed to withdrawal from this study at any time without showing any causes.
Sampling procedure
The simple random sampling procedure was used for sampling.
Inclusion criteria
Patients with symptoms of inflammatory polyarthritis with history < 2 years duration; and not fulfilling diagnostic criteria of ACR criteria or EULAR criteria for any other rheumatological conditions at initial presentation were included.
Exclusion criteria
Patients who have already received disease-modifying antirheumatic drugs (DMARDs) for any reason were excluded.
Clinical assessment
After taking informed consent from all patients, detailed history regarding the onset, progression, family history, presence of extra-articular features, distribution of joints presenting with arthritis were obtained, and documented in the preset pro forma. Morning stiffness was pointed by patients on a visual analog scale (range: 0–100 mm); the duration of morning stiffness was also assessed. A 66-joint count for the swollen joint count (SJC) and tender joint count (TJC) were performed so also routine blood examination including C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and RF as well as anti-CCP antibody (antibody against citrullinated peptide) were performed as baseline investigations.
Patients were evaluated at baseline and every 3 months according to prediction rule derived from Leiden Early Arthritis Clinic that includes - TJC and SJC, laboratory indices and disability measured by the Health Assessment Questionnaire and assessment of disease activity by the Disease Activity Score 28 (DAS28) and baseline photographs of small joints of both hands. Fifty-eight patients were enrolled. Eight patients were lost to follow-up and were excluded from final analysis.
The prediction rule consists of clinical variables, which are scored (range: 0–13) and corresponds to the percent chance of developing RA. The rule was applied to baseline characteristics of all patients with UA, who had completed 1-year follow-up to allow sufficient time for diagnosis. After 1 year, all patients were examined to determine if RA or another ACR-defined rheumatological condition had developed or the disease course persisted as UA or had a complete remission (defined as DAS28 ≤2.6).
A small sample size (15 numbers) pilot study was conducted to check the content and appropriateness of the questionnaire. Large sample internal and external validity test were not performed.
Statistical analysis
Collections of data are analyzed, and statistical tests are done with the help of Microsoft Excel, Statistical Package for the Social Sciences (SPSS) /Version 14, Developer: IBM (Chicago, IL, USA).
Patients were divided into two groups: Those who developed RA after 1 year of follow-up and those who did not. Mann–Whitney U-test was performed for ordinal variables (e.g., SJC) and t-test was performed for continuous variables (e.g., CRP). Among the variables that showed significant change, TJC, duration of morning stiffness, and duration of arthritis were selected as clinical variables for linear discriminant analysis with disease outcome being the dependent variable. These variables satisfied prerequisites for the analysis. Discriminant scores (D) for each patient was calculated. A receiver operating characteristic (ROC) curve was constructed with the discriminant score and compared with Leiden prediction score.
Results
Table 1 describes the characteristics of fifty UA patients satisfying inclusion criteria. The mean age was 40.3 years and most was female 82% (41/50). All patients had been followed up for 1 year. At follow–up, 54% (27/50) patients were diagnosed with RA, whereas the remainder 46% (23/50) developed other rheumatologic condition or inflammatory arthritis due to viral infection, remained undifferentiated or attained complete remission. Of the patients who did not progress to develop RA (nonprogressors), 2% (1/50) patient was diagnosed with diffuse scleroderma due to subsequent development of skin and other characteristics of the disease as well as Scl-70 positivity. About Eight percent (4/50) patients developed symptoms consistent with postviral arthritis and 4% (2/50) patients with systemic lupus erythematosus (SLE). The largest group (n = 13, 26%) of nonprogressors remained undifferentiated at follow-up.
| No Progression to RA (n=23) | Progression to RA (n=27) | P | ||||
|---|---|---|---|---|---|---|
| Age, mean (SD) years | 40.3 (10.4) | 36.7 | (10.4) | 0.22 | ||
| Sex, F: M | 16:7 | 25: 2 | ||||
| Swollen joint count, median | 6 (0-14) | 8 (0-16) | P<0.001 | |||
| Tender joint count, median | 17 | (6-28) | 18 | (8-38) | 0.005 | |
| RF+ve (percent) | 2 | (8.7) | 14 | (51.9) | 0.001 | |
| Anti CCP titre, mean ( SD) | 0.65 (0.2) | 4.6 | (7.9) | <0.02 | ||
| ESR, mean( SD ) mm 1st hour | 54.7 ( 19.6) | 58.3 | (22.6) | 0.55 | ||
| CRP, mean (SD) mg/L | 21.9 ( 8.3) | 19.5 ( 13.9) | 0.47 | |||
| Morning Stiffness, mean (SD) minutes | 33 | (29.9) | 87.8 | (49.9) | P<0.001 | |
| Symptom duration | ||||||
| < 1 month (percent) | 10 | (43.5) | 1 | (3.7) | P<0.01 | |
| 1-3 month (percent) | 6 (26.0) | 13 | (48.2) | |||
| > 3 month (percent) | 7 (30.5) | 13 | (48.1) | |||
| DAS 28 mean ( SD) | 5.66 (1.01) | 6.86 (82) | P<0.001 | |||
Table 1: Baseline characteristics of patients with undifferentiated arthritis
Patients progressing to RA were more likely to have a positive family history of RA and present with symmetric joint involvement initially. Patients who developed RA also had longer duration of morning stiffness and high inflammatory markers although it was not statistically significant. Factors significantly associated with progression to RA were the TJC and SJC, RF positivity, anti-CCP positivity, high DAS28, longer symptom duration at first presentation as well as longer duration of morning stiffness.
Table 2 shows the number of patients who developed RA in relation to the calculated prediction score, the regression coefficients of the predictive variables were rounded to the nearest number ending in 5 or 0. No patients with UA, who scored 4 or less progressed to RA, whereas all who scored 7 or more, did progress]. For those who scored between 4 and 7, higher scores frequently predicted progression [Table 3]. Among progressors, the median prediction score was 9.20 (interquartile range [IQR] 5.52–10.70) while nonprogressor’s median score was 5.70 (IQR 2.36–7.2). Thirty-three (66%) patients with UA scored between 5 and 8, and they had confirmed RA by 1-year follow-up.
| Prediction score | No progression to RA (n=23) | Progression to RA (n=27) |
|---|---|---|
| 0 | 0 (0) | 0 (0) |
| 1 | 0 (0) | 0 (0) |
| 2 | 2 (100) | 0 (0) |
| 3 | 0 (0) | 0 (0) |
| 4 | 3 (100) | 0 (100) |
| 5 | 3 (75) | 1 (25) |
| 6 | 12 (70.6) | 5 (29.4) |
| 7 | 3 (37.5) | 5 (62.5) |
| 8 | 0 (0) | 4 (100) |
| 9 | 0 (0) | 3 (100) |
| 10 | 0 (0) | 6 (100) |
| 11 | 0 (0) | 3 (100) |
| 12 | 0 (0) | 0 (0) |
| 13 | 0 (0) | 0 (0) |
| Median score (IQR) | 5.70 (2.36-7.2) | 9.20 (5.52-10.70) |
Table 2: A. Prediction score distribution according to disease outcome (First Visit)
| Cut-off value | No progression to RA | Progression to RA |
|---|---|---|
| <4.0 | 2 (100) | 0 (0) |
| 4.0-7.0 | 21 (65.6) | 11 (34.3) |
| >7.0 | 0 (0) | 16 (100) |
Table 3: Cut-off values for prediction scores and risk of development of RA on first visit
Mann–Whitney U-test was performed to calculate significance level of SJC and TJC. Significance levels were P < 0.001) and P < 0.01, respectively.
Unstandardized canonical discriminant coefficients for TJC (T), duration of morning stiffness (M), and duration of arthritis (A) were 0.164, 0.066, and 0.012, respectively. The constant was calculated as −2.838. ROC curve was plotted with the formula:
D = 0.164 × T + 0.066 × M + 0.012 × A − 2.838.
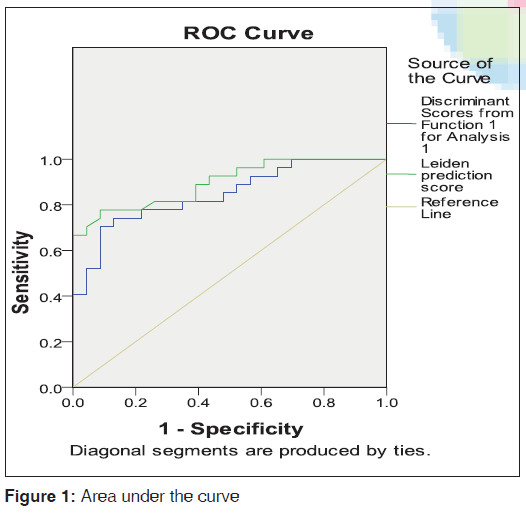
Area under curve (AUC) at 95% confidence interval for our discriminant function was 0.845 (standard error [SE] 0.054). In comparison, AUC of Leiden prediction score was 0.897 (SE 0.043) [Figure 1].
Discussion
RA is the most common inflammatory arthritis, affecting 0.5–1% of the general population.[9] Prevalence of RA is about 0.075% in India.[10] Over the past decade, treatment of RA has been characterized by early, aggressive treatment with DMARDs, because this treatment strategy prevents joint damage and functional disability.[11] In rheumatology clinics, the majority of patients who present with recent onset arthritis have UA, which is a form of arthritis that does not fulfill the classification criteria for a more definitive diagnosis.
With regard to early UA, it was observed that predictive factors for the fulfillment of the 2000 EULAR criteria for RA and for having persistent arthritis were largely similar. Perhaps, of greater clinical utility is the DAS, which provides a composite measure of disease activity based on joint counts ESR and patient global assessment of general health and is also easier to use in the clinics.[12] However, this composite score (DAS) is just a measure of disease activity and cannot predict the development of RA among UA patients. A group of patients from the Amsterdam early arthritis clinic with peripheral arthritis and disease duration of < 3 years were followed in order to identify independent predictors of outcome.[13] In this study, 27% of the patients were clinically diagnosed as having UA at inclusion and 72% as RA. After 1-year follow-up, 42% of the patients were diagnosed as RA, but no individual predictor was found. Leiden Early Arthritis Clinic included patients with any form of arthritis < 2 years duration confirmed by a rheumatologist. Out of 936 patients at inclusion, 37% were categorized as having UA, and 22% were diagnosed with RA. After 1 year of follow-up, 32% of the UA patients fulfilled the ACR 1987 criteria for RA. The percentage increased to 40% at 3 years of follow-up.[14] Validation and modification of original cohorts derived in Leiden show 100% of patients with a score 8.0 had progressed to RA, whereas 94% of patients with a score 6.0 did not develop RA.[3] Further studies showed that predictors for RA development, previously used to develop the Leiden prediction rule in UA patients, are largely similar to predictors for arthritis persistency.[15] Recently, in a small pilot study in a Canadian cohort of early UA, the validation of the score revealed that 72% patients with score < 5 did not develop RA and 97% with score >8 did develop RA. In India, a small sample cohort of early arthritis was followed over 3 months using Leiden prediction rule for predicting development of RA, and it showed that this rule was not validated in Indian cohorts. However, in our study, we found that Leiden prediction rule is a fast and easy tool to help identify patients with UA, who may go on to meet criteria for RA in future. Baseline scores >7 predicted those who developed RA by 12 months. From this study, it is clear that Indian patients with a predictive score of 7 or more are clearly associated with the development of RA in 100%, whereas those with a score in between 4 and 7 has a major chance of 70.6% of remission or other diagnosis at 1-year follow-up. A score of 4 or less is also clearly nullifying the possibility of future development of RA at 1 year.
These findings resemble the original model, where scores of < 6 and above 8 most accurately predicted outcome. Similar to the Leiden derivation cohort, the number of tenders and swollen joints, RF positivity, anti-CCP positivity, and poor functional status predicted the development of RA. Specifically, in Indian cohorts, a value of 7 or more is associated with the outcome of RA in comparison to the Western studies. The discrimination value of the ROC curve analysis, shown by the AUC was 0.897 (SE 0.043).
In search of a more simplified prediction formula applicable to the same cohort, we have developed a formula, using only three clinical parameters from Leiden’s original nine variables. ROC curve was plotted with the formula:
D = 0.164 × T + 0.066 × M + 0.012 × A − 2.838.
TJC (T), duration of morning stiffness (M), and duration of arthritis (A) was 0.164, 0.066, and 0.012, respectively. The constant was calculated as −2.838.
A score of < 0 means patients will not develop RA whereas a positive value means progression to RA in future follow-up. ROC curve analysis of our discriminant function, with AUC at 95% confidence interval was 0.845 (SE 0.054). In comparison, AUC of Leiden prediction score was 0.897 (SE 0.043) [Figure 1], which is very much comparable. This simpler version of prediction model may help us in developing world where costly investigations always jeopardise treatment and diagnosis. However, before coming to conclusion, a large multi-center study is needed with larger cohort for both internal and external validation.
The major limitation of our study is of small sample size, and it differs from the derivation cohort of Leiden original prediction in three major ways. First, a large proportion of our patients with UA (54%) on follow-up developed RA, compared with 31% of the Leiden cohort. Later, 8% patients were found to be suffering from postviral arthritis, namely dengue and chikun gunya fever, which are common diseases in Southeast Asian countries but were not studied in Leiden. About 4% of patients were later diagnosed as SLE according to ACR criteria. A single female patient subsequently developed scleroderma whose initial presentation was very similar to early RA. These differences may reflect our inclusion criteria favoring types of inflammatory arthritis, such as RA, based on presenting signs and symptoms. In contrast, patients with any physical examination evidence of arthritis are enrolled in the Leiden clinic and, therefore, may encompass more benign forms of arthritis or even self-limiting disease. A large multi-center study involving more patients with early arthritis is needed to support this pilot study and also validate the prediction formulation we have derived.
Duration of morning stiffness at presentation was found to have significant correlation to future development of RA at 1 year though it has been excluded from recently proposed EULAR/ ACR criteria of RA classification. Symptom duration at first visit to our center has a positive prediction for RA. Forty percent of our patients present after 3 months of their onset of symptoms, of these 48.1% develop RA in 1-year follow-up. This indicates that a large number of patients come to us after the loss of window of opportunity when erosive damage has already expected to have occurred.
Conclusion
Leiden score is partially valid in the Indian patients with UA and larger study is needed to validate the formulation taking only clinical variables of original Leiden prediction model.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| Test result variable(s) | Area | Standard errora | Asymptotic significanta | Asymptotic 95% confidence interval | |
|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||
| Discriminant scores from function 1 for analysis 1 | 0.845 | 0.054 | <0.001 | 0.739 | 0.952 |
| Leiden prediction score | 0.897 | 0.043 | <0.001 | 0.813 | 0.981 |
| aNull hypothesis: True area=0.5 | |||||
References
- Symmons DP, Jones MA, Scott DL, Prior P. Longterm mortality outcome in patients with rheumatoid arthritis: Early presenters continue to do well. J Rheumatol 1998;25:1072-7.
- Pratt AG, Lorenzi AR, Wilson G, Platt PN, Isaacs JD. Predicting persistent inflammatory arthritis amongst early arthritis clinic patients in the UK: Is musculoskeletal ultrasound required? Arthritis Res Ther 2013;15:R118.
- van der Helm-van Mil AH, le Cessie S, van Dongen H, Breedveld FC, Toes RE, Huizinga TW. A prediction rule for disease outcome in patients with recent-onset undifferentiated arthritis: How to guide individual treatment decisions. Arthritis Rheum 2007;56:433-40.
- van Dongen H, van Aken J, Lard LR, Visser K, Ronday HK, Hulsmans HM, et al. Efficacy of methotrexate treatment in patients with probable rheumatoid arthritis: A double-blind, randomized, placebo-controlled trial. Arthritis Rheum 2007;56:1424-32.
- Kallberg H, Padyukov L, Plenge RM, Ronnelid J, Gregersen PK, van der Helm-van Mil AH, et al. Gene-gene and gene-environment interactions involving HLA-DRB1, PTPN22, and smoking in two subsets of rheumatoid arthritis. Am J Hum Genet 2007;80:867-75.
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis.Arthritis Rheum 1988;31:315-24.
- Cader MZ, Filer A, Hazlehurst J, de Pablo P, Buckley CD, Raza K. Performance of the 2010 ACR/EULAR criteria for rheumatoid arthritis: Comparison with 1987 ACR criteria in a very early synovitis cohort. Ann Rheum Dis 2011;70:949-55.
- Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569-81.
- Mochan E, Ebell MH. Predicting rheumatoid arthritis risk in adults with undifferentiated arthritis. Am Fam Physician 2008;77:1451-3.
- Chandrasekaran AN, Radhakrishna B. Rheumatoid arthritis and connective tissue disorders: India and South-East Asia. Baillieres Clin Rheumatol 1995;9:45-57.
- Pedersen M, Jacobsen S, Garred P, Madsen HO, Klarlund M, Svejgaard A, et al. Strong combined gene-environment effects in anti-cyclic citrullinated peptide-positive rheumatoid arthritis: A nationwide case-control study in Denmark. Arthritis Rheum 2007;56:1446-53.
- van der Heijde DM, van’t Hof M, van Riel PL, van de Putte LB. Development of a disease activity score based on judgment in clinical practice by rheumatologists. J Rheumatol 1993;20:579-81.
- Gärtner M, Fabrizii JP, Koban E, Holbik M, Machold LP, Smolen JS, et al. Immediate access rheumatology clinic:Efficiency and outcomes. Ann Rheum Dis 2012;71:363-8.
- van Gaalen FA, Linn-Rasker SP, van Venrooij WJ, de Jong BA, Breedveld FC, Verweij CL, et al. Autoantibodies to cyclic citrullinated peptides predict progression to rheumatoid arthritis in patients with undifferentiated arthritis: A prospective cohort study. Arthritis Rheum 2004;50:709-15.
- Kuriya B, Cheng CK, Chen HM, Bykerk VP. Validation of a prediction rule for development of rheumatoid arthritis in patients with early undifferentiated arthritis. Ann Rheum Dis 2009;68:1482-5.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.