Analysis of Oxidative Stress in Patients with Breast Cancer and Obesity
2 Department of Medicine and Pharmacy, University of Medicine and Pharmacy, Bucharest, Romania
3 Department of Radiotherapy, Institute of Oncology, Bucharest, Romania
4 Department of Surgery, Dr Ion Cantacuzino Hospital, Bucharest, Romania
5 Department of Oncology, Elias Hospital, Bucharest, Romania
6 Research Department, Institutue of Oncology, Bucharest, Romania
Department of General Surgery, Clinical Emergency Hospital, Bucharest, Romania
, DOI: 10.54608.annalsmedical.2021.9
Citation: Ali Qasim MS, et al. Analysis of Oxidative Stress in Patients with Breast Cancer and Obesity. Ann Med Health Sci Res. 2021;11:1578-1585.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: Breast cancer is a major source of public health concern. Our work aims to study the role of oxidative stress in breast pathophysiology. Materials & Methods: A group of 39 patients diagnosed and treated for breast cancer was followed in a prospective, observational, nonrandomized study to assess the serum oxidative stress levels. Thus, the following parameters were determined: Malondialdehyde (MDA) concentration as a measure of lipid peroxidation reaction, serum thiol concentration as end products of oxidative degradation of proteins, and total level of antioxidants. Results: 81.9% of these patients have an excess of adipose tissue (overweight or obesity). A positive correlation was obtained between the serum peroxides values and obesity as measured by Body Mass Index (BMI) (p<0.01) and waist circumference (p<0.05). The mean values of serum thiols were higher in obese patients versus non-obese ones (341 μmol/l vs. 336 μmol/l), but without statistical significance. Regarding the influence of obesity on antioxidants levels, no statistically significant results were obtained. Conclusion: Breast cancer patients had significantly high levels of oxidation markers such as MDA and low levels of antioxidant markers, respectively thiols and total antioxidants. The obtained oxidative stress parameters are independent of obesity and are mainly related to the presence of breast cancer.
Keywords
Breast cancer; Oxidative stress; Obesity; Serum peroxide; Antioxidant markers
Introduction
Breast cancer is a major source of public health concern. The latest data published by the International Agency on Cancer Research (IARC) report that 110.000 (23.6%) cases of the obesity related cancers are attributed to breast cancer in postmenopausal patients, also suggesting that at least 28.000 of those could have been prevented. [1] The results of a metaanalysis from 2014 on 200.000 patients diagnosed and treated for breast cancer included in 82 clinical trials concluded that patients with a high Body Mass Index (BMI) had lower survival rates, compared to those with normal BMI. The same metaanalysis also reported that other individual prognostic factors such as menopausal status or hormonal receptors status were not found to be related to the survival rates. [2]
After molecular testing, three mechanisms were identified to justify the relationship between the excess of adipose tissue and the development of a tumor favorable microenvironment. The first would be an increase in the synthesis of cytokines triggering the cascade of inflammation, with the onset of a chronic inflammatory status. Secondly, hyperinsulinemia, as a consequence of increased insulin resistance, was also incriminated in cancer development due to increasing levels of circulating Insulin-like Growth Factor (IGF), an important proangiogenic and antiapoptotic factor. The third mechanism would be hyperplasia and hypertrophy of fat cells, with the installation of a hypoxic state, causing increased levels of oxidative stress, stimulation of angiogenesis, increased hypoxiainducible factor-1 expression thus promoting angiogenesis and tumor development. [3]
Due to their high prevalence rate worldwide, breast, cervical and ovarian cancers were intensively investigated for identifying treatment response and prevalence prognostic factors. Environmental, hormonal, viral, but also stage and treatmentrelated factors were linked to the development or treatment response of these tumors. [4-9]
The development and progression of breast cancer have been linked to the interaction between tumor cells and the tumor microenvironment. How the tumor microenvironment is altered by the level of oxidative stress is considered an essential molecular mechanism that explains why the adipose tissue influences the prognosis of breast cancer. [10] Oncobiology studies data lead to the conclusion that breast carcinoma should not be seen as an isolated group of cells that have undergone mutations, but as a microenvironment consisting of breast cancer cells, fibroblasts, adipocytes, immune and endothelial cells. [11] The interactions between cancer cells and stroma are involved in regulating signaling pathways in the evolution of this tumor. One of the most important factors influencing the development, progression and metastasis of cancer is oxidative stress. Reactive Oxygen Species (ROS) interact with various cellular components such as proteins, lipids, and nucleic acids resulting in structural and functional alterations of these components, thus further leading to an irreversible disruption of the cellular functions. Damage to DNA, proteins, cell membrane and mitochondria are involved in carcinogenesis, although no specific biochemical markers have yet been identified. In addition, information on biochemical changes in tissues and blood, especially antioxidant status, and its correlation with the clinical stage of the disease, is lacking.
The oxidant-antioxidant balance in the tissues is believed to aid the development and progression of cancer. Previous research has shown changes in this balance during the colorectal oncogenic process. [9]
In Romania, breast cancer is the leading cause of death. To this end, we are interested in this work a relationship between breast cancer, redox status and obsession.
Materials and Methods
The study is prospective, observational, non-randomized, performed on a sample of 39 patients, evaluated between January 2019 and October 2019 at Elias University Emergency Hospital, a representative sample for a population of patients diagnosed with breast cancer in a center with experience in the diagnosis and treatment of oncological diseases.
The database was completed using information from the patient’s medical file, as well as laboratory results obtained by dosing the parameters of oxidative stress in the venous blood. Thus, recorded data were represented by BMI, waist circumference and serum levels of Malondialdehyde (MDA), thiols, and total antioxidants.
All patients signed an informed consent for data processing and for biological sampling (5 ml venous blood). Also, the approval of the Institutional Ethics Commission (no. 5748/13.08.2018) was obtained for conducting the study.
The inclusion criteria in the study were represented by
Patients diagnosed with stage I, II, or III invasive ductal or lobular carcinoma undergoing chemotherapy in the adjuvant or neoadjuvant setting or monitoring for the first two years after adjuvant or neoadjuvant chemotherapy The absence of another associated type of neoplasia Patients for whom all the information necessary to complete the database were available Patients were included in the study in the order of hospitalization for chemotherapy. They were in different stages of treatment: Adjuvant chemotherapy, neoadjuvant chemotherapy, adjuvant endocrine therapy or radiation therapy, but all of these patients underwent adjuvant or neoadjuvant chemotherapy in the first two years after diagnosis.
The evaluation of patients consisted of collecting 5 ml of venous blood, as well as filling in a patient evaluation form.
We aimed to identify a correlation between excess adipose tissue assessed by calculating BMI and measuring waist circumference with serum levels of oxidative stress parameters.
Determination of oxidative stress parameters
To assess the level of oxidative stress in the previously presented group, serum parameters related to the oxidative attack on lipids, proteins and the determination of the level of activation of endogenous defense systems were evaluated. All determinations were performed according to standardized techniques, processed from the serum collected from the patients included in the study.
Lipid peroxidation was assessed by measuring the serum concentration of MDA by the Carbonneau spectrophotometric method. This method is based on the production of a colored adduct (MDA-TBA2) with a maximum absorption at 532 nm depending on the concentration. [12,13] Normal values are between 0 μmol-4 μmol/100 ml serum.
SH-albumin thiol groups were measured by reaction with Ellman's reagent (5,5 dithiobis-2-nitrobenzoic acid) which reached a maximum intensity at 412 nm according to SHgroups. [14] Normal levels range are from 370 μmol/ l-450 μmol/l.
Total antioxidants were measured based on the ability of the serum to reduce iron (at low pH, the FeIII-tripyridyl-s-triazine complex is reduced to the ferrous state and forms an intense blue complex, and the maximum color intensity is at the length of 593 nm wave). [15] Normal values are between 0.9 μmol/l-1.4 μmol/l.
Statistical analysis
The R program version 3.6.2 (2019) was used for statistical analysis. In addition to the standard packages, the following packages have also been used:
Survival Therneau T (2015). A package for survival analysis in S. Version 2.38, <URL:https://CRAN.R-project.org, Alboukadel Kassambara, and Marcin Kosinski (2018)
Survminer: Drawing Survival Curves using ggplot2. R package version 0.4.3. https://CRAN.R-project.org
In this study, BMI and abdominal circumference were used as indicators of obesity. The analysis algorithm consisted in estimating the possible correlations between BMI/abdominal circumference and oxidative stress evaluation parameters, using the correlation index r Pearson/ρ Spearman, then making a comparative analysis by batches of these indices (obese vs. nonobese), using depending on the distributions of the variables either a bidirectional Welch t test for two independent samples, either a Wilcoxon Rank Sum test.
Results
The characteristics of the patients included in the studied group are presented in Table 1.
| Table 1: Patient related characteristics. | |
|---|---|
| Characteristic | Number and percentage of patients |
| Age at diagnosis | |
| <50 years | 13 (33.3%) |
| 50-70 years | 22 (56.4%) |
| >70 years | 4 (10.2%) |
| BMI | |
| 18-25 kg/sqm | 7 (17.9%) |
| 25-30 kg/sqm | 14 (35.8%) |
| >30 kg/sqm | 18 (46.1%) |
| Waist circumference | |
| <80 cm | 4 (10.2%) |
| 81-88 cm | 6 (15.4%) |
| >88cm | 29 (74.4%) |
| Intrinsic subtype | |
| Luminal A | 8 (20.5%) |
| Luminal B | 19 (48.7%) |
| Luminal B Her2 positive | 5 (12.8%) |
| Her2 positive | 2 (5.2%) |
| Triple negative | 5 (12.8%) |
| Stage T | |
| T1 (<2 cm) | 7 (18%) |
| T2 (2-5 cm) | 18 (46%) |
| T3 (>5 cm) | 1 (2.6%) |
| T4 (invasion of the chest wall or skin) | 13 (33.4%) |
| Invasion of regional lymph nodes | |
| Present | 30 (77%) |
| Absent | 9 (23%) |
It was a higher percentage for patients for patients diagnosed with breast cancer in the 50-70 age range most of the patients presented in T2 and T4 stages, and the most common subtype encountered was luminal B.
Although in this group the patients were not selected according to the BMI, there are a significant percentage of overweight and obese patients. We note that the percentage of obese patients is 46.1%, respectively 35.8% for the overweight ones. Thus, 81.9% of the investigated patients have excess of adipose tissue. We mention that the height and weight taken into account were those from the diagnosis. The waist circumference at the time of signing the informed consent was greater than 88 cm in most patients (74.4%) [Table 1].
Analysis of the influence of obesity on lipid peroxides levels
As is well known, because lipids have a large distribution in the body and in the cell membrane, the first attack of oxygen free radicals is at this level.
To perform this analysis, lipid peroxides levels were determined in the serum of the patients included in the study according to the method presented in the materials and methods section. The Pearson correlation index was determined between the calculated obesity parameters (BMI/waist circumference) and serum levels of lipid peroxides. The results obtained [Table 2] showed a statistically significant positive average correlation (p<0.01) between BMI and peroxides value; there was also a statistically weak positive mean correlation (p<0.05) between the waist circumference and biochemically determined value of lipid peroxides.
| Table 2: Calculation of the Pearson correlation index between obesity (BMI/waist circumference) and serum peroxides. | |
|---|---|
| Obesity parameter | Peroxide sr Pearson (Value p) |
| BMI | 0.436 (0.0061) |
| Waist circumference | 0.338 (0.0379) |
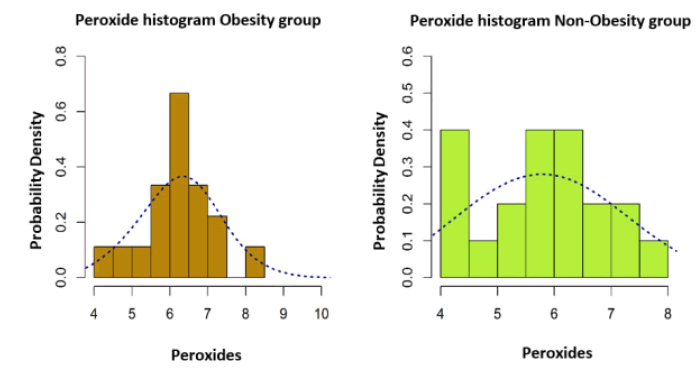
The serum peroxide values measured in the studied patients and presented in Table 3. Areincreased compared to those considered within normal limits.
| Table 3: Descriptive analysis of the level of peroxides on the two lots. | ||
|---|---|---|
| Peroxides (μmol/100ml) | Obesity | Non-Obesity |
| Mean ± S.D | 6.25 ± 0.86 | 5.81 ± 0.99 |
| Median (IQR) | 6.28 (1.00) | 5.92 (1.51) |
| Min to Max | 4.48 la 8.13 | 4.39 la 7.70 |
| Skewness | -0.08 | 0.14 |
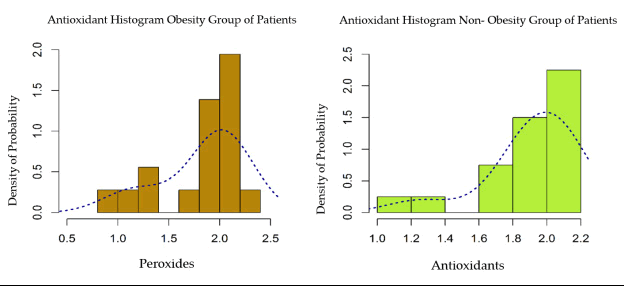
The data presented in the histograms in Figure 1 represent the values of the correlation between BMI and the intensity of the lipid peroxidation reaction. After analyzing these histograms, a distribution model close to the normal distribution is highlighted, which is why a bidirectional Welch test was applied to two independent samples.
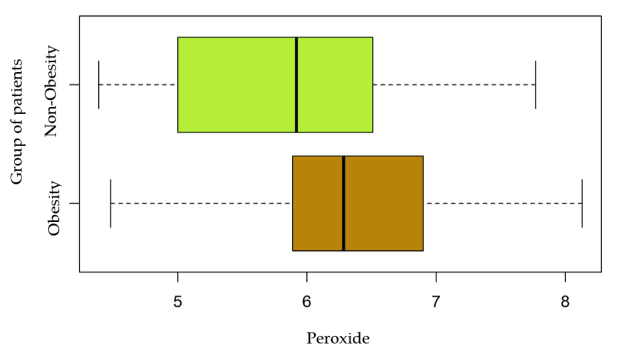
The differences between the values obtained are without statistical significance (p>0.05), but as shown in the graph in Figure 2, there is a change in the mean values of lipid peroxides in the group of obese patients, compared to lower values in those without metabolic changes.
Thus, the values of the final product of the lipid peroxidation reaction are not statistically significantly modified in obese versus non-obese patients, but there are small numerical differences that may represent an increase in lipid metabolism and may direct their peroxidation [Figure 3].
Analysis of the influence of obesity on thiol levels
After initiating chain reactions, excess ROS attack protein structures, inducing a number of molecular, structural and functional changes at this level.
In the next stage of the study, we correlated the values of obesity parameters with the level of thiols obtained from oxidative degradation reactions of proteins.
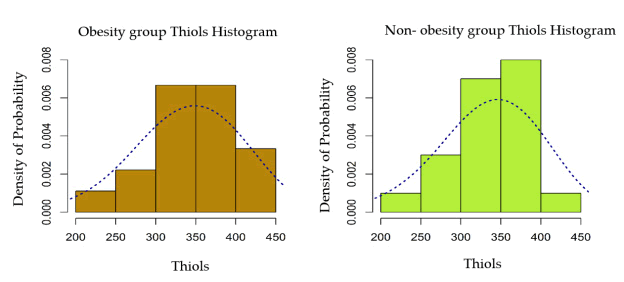
According to Table 4 which illustrates the mean and median serum thiol values in the two groups of obese/non-obese patients respectively, the results obtained do not correlate with statistical significance (p>0.05).
| Table 4: Descriptive analysis of thiols on the two lots. | ||
|---|---|---|
| Thiols (µmol/l) | Obesity | Non-Obesity |
| Mean ± D. S | 341.27 ± 50.61 | 336.65 ± 48.74 |
| Median (IQR) | 349.50 (80.00) | 336.50 (72.00) |
The two distributions were tightened and a bidirectional Welch test is used for comparison for two independent samples. The difference is without statistical significance (p>0.05).
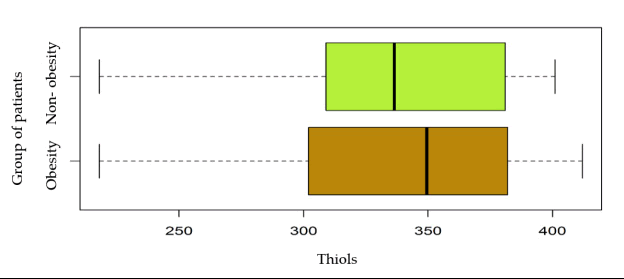
The graph in Figure 4 does not show significant differences between serum thiol levels in obese patients compared to nonobese patients.
Analysis of the influence of obesity on antioxidants levels
Because there are data in the literature that support the existence of an antioxidant defense deficiency in oncological patients, we tried to identify whether excess adipose tissue influences the serum level of antioxidants. Table 5 describes the characteristics of the two groups of obese/non-obese patients, depending on the serum levels of total antioxidants.
| Table 5: Descriptive analysis of the level of antioxidants for the 2 batches. | ||
|---|---|---|
| Antioxidants (µmol/l) | Obesity | Non-Obesity |
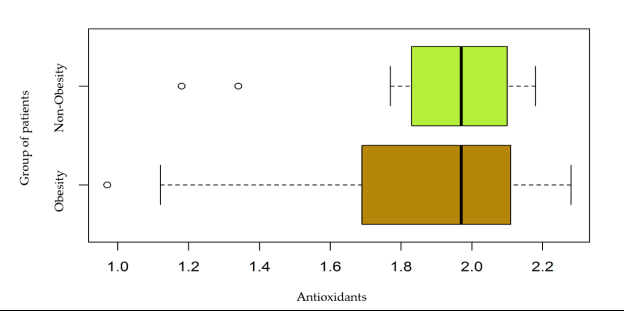
| Mean ± S.D | 1.82 ± 0.39 | 1.91 ± 0.25 |
| Median (IQR) | 1.97 (0.42) | 1.97 (0.27) |
| Min la Max | 0.97 la 2.28 | 1.18 la 2.18 |
| Skewness | -1.09 | -1.64 |
Thus, the Pearson correlation index was evaluated in obese patients compared to the normal-weight ones and the histograms of the two distributions are shown in Figure 5.
Histogram of serum antioxidant distribution within the two groups (obese vs. non-obese patients) Both distributions were characterized by a significant negative asymmetry. A Wilcoxon Rank Sum test was used for comparison.
The differences were without statistical significance (p>0.05). The graph shown in Figure 6 does not support a difference in serum antioxidants levels between obese and non-obese patients.
Discussion
This research paper was designed for clarifying the role of oxidative stress in influencing the prognosis of obese patients diagnosed with breast cancer. Both breast cancer and obesity are associated with disorders of the homeostasis of ROS, but how oxidative stress parameters influence the tumor microenvironment and subsequently the prognosis of these patients is still investigated. [16]
Obesity was quantified by measuring both BMI and waist circumference in the context of studies supporting the role of abdominal obesity in increasing the risk of breast cancer. [17]
The evaluation of the oxidative stress was made by serum investigation of some parameters involved in the oxidative attack on lipids, proteins and the determination of the activation level of the endogenous antioxidant defense systems. In this regard, serum concentration of MDA was determined as a measure of the lipid peroxidation reaction, serum thiol concentration as a final product of oxidative degradation of proteins and, in particular, of those containing sulfur and serum level of total antioxidants was measured too.
Given the role of oxidative stress in the pathogenesis of breast cancer, Pala et al. suggested that the disease is characterized by "pro-oxidants" that alter the redox state of thiol/disulphide and affect glucose tolerance, generating the so-called " mitochondrial oxidative stress.” This means that the intracellular thiol/disulphide state is moved to an oxidative state, due to the persistent creation of a large number of ROS. [18]
Regarding MDA, a statistically significant positive mean correlation was identified between BMI and serum value of lipid peroxidation compounds (p<0.01). This correlation is supported by the hypothesis that adipose tissue, by maintaining a high degree of systemic inflammation and increased activity of macrophages, leukocytes and polymorphonuclear cells, causes excess production of ROS with a proportional increase in oxidative stress.
Another significant aspect is the identification of an increased serum level of peroxides in the studied patients, compared to the serum values considered normal. Thus, compared with the value considered normal 0 μmol-4 μmol/100 ml serum, in obese patients the average value was 6.25 μmol/100 ml and in non-obese patients it was 5.81 μmol/100 ml. These values are considered in the context of a lipid peroxidation reaction accentuated by the presence of neoplastic disease.
Data from the literature mention that lipid peroxidation, an intensely process once initiated, occurs as a chain reaction. Obesity can decrease the incidence of breast cancer in women through a specific mechanism that involves generation of lipid peroxidation products. [19] So, lipid peroxidation an episodic phenomenon involved in apoptosis, the cell cycle for the development and differentiation of tumor cells provides some protection in breast cancer. [20,21] This consideration of lipid peroxidation as a protective factor in breast cancer does not contradict the conventional view that oxidative degradation of lipids is an undesirable cytotoxic process, but emphasizes the protective phenomenon only in one stage. [20] This may explain the values obtained in this study, widely distributed, significantly not correlated with the parameters of obesity. Data from the literature indicate that the associations between dietary factors and breast cancer remain controversial, but several results suggest that lipid peroxidation may have a protective effect in patients with breast cancer and metabolic disorders, such as obesity. [22]
In the context that visceral obesity quantified by measuring waist circumference has an influence on increased mortality and morbidity, [23] this study sought to demonstrate a correlation between increased serum lipid peroxidation levels and waist circumference. By calculating the Pearson correlation index, a statistically significant weak positive correlation is found (p<0.05). Data from the literature, such as this research, indicate that the determination of lipid peroxidation compounds could be a valuable tool in assessing the prognosis of breast cancer, requiring additional studies to standardize the methodology. [24] The determination of serum thiols evaluated the antioxidant barrier of proteins in normal weight patients compared to obese ones. Although mean and median values were slightly increased in the group of obese patients compared to those of normal weight, this difference was statistically insignificant (341 μmol/l vs. 336 μmol/l). However, lower values are obtained compared to normal ranges (370 μmol/l-450 μmol/l).
The Sulfhydryl (SH) groups in the composition of thiols act as a substrate for antioxidant enzymes, but they also have the role of blocking free radicals. In the human body, these-SH groups are a component of albumin mainly, but there also components of low molecular weight proteins such as cysteine, cysteinyl glycine, glutathione, homocysteine and γ-glutamyl cysteine. Under oxidative stress, thiol groups form disulfide bonds which can be reduced back to thiol groups that reenter the circuit and thus thiol-disulfide homeostasis is maintained. A study from 2019 conducted on a number of 37 patients with breast cancer compared to 31 healthy patients in which the values of serum thiols were analyzed, but also of compounds containing thiol disulfide groups, showed a statistically significant difference between the two groups in terms of serum concentration of groups containing thiol-sulfide groups. [25]
Endocrine functions, altered in obese patients due to the accumulation of visceral adipose tissue become important sources of oxidative stress manifested by decreased antioxidant capacity. The oxidant-antioxidant balance is necessary to maintain optimal physiological conditions in the body. Thiolmediated redox mechanisms involve antioxidant reactions. The disulfide bridges between two cysteine amino acids regulate protein oxidation. An increase in thiol levels or thiol/disulfide ratios represents the intensity of antioxidant protection. Dynamic homeostasis of the thiol-disulfide ratio is necessary for antioxidant protection, regulation of enzymatic activity and detoxification, being also involved in the pathogenesis of various chronic diseases, such as diabetes, cardiovascular disease and cancer. [26]
Lipid peroxidation products, including MDA, are commonly used as biomarkers of oxidative stress because they may contribute to or reflect the amplification of cellular damage resulting from the generation of oxidized compounds. In addition, thiols, resulting from glutathione, cysteine and cysteine glycine, are natural reservoirs with reducing power and act as intracellular and extracellular redox buffers. [27]
Of all antioxidants, thiols are a major defense against ROS. The redox states of thiols play a critical role in determining the structure and function of proteins, regulating the enzymatic activity of transcription factors and antioxidant protection. Oxidative stress results from the imbalance created between the excess of ROS and the decrease of the antioxidant barrier.
For the complete evaluation of the oxidative stress parameters in this study, the serum level of total antioxidants in the study group was evaluated to assess a possible difference between obese patients compared to normal weight ones. The results do not support a statistically significant difference between the two groups of patients, thus do not demonstrate a benefit of antioxidant therapy in the prevention of breast cancer [28] or as an additive treatment in the therapeutic approach of this type of cancer. [29]
Cancer is a dynamic, multi-factorial and intrinsically complex disease. Despite these aspects, the tumor growth environment in each patient is much more stable and uniform, because most of the factors in this environment come from the predictable determinants of the patient's physiology. Thus, targeting this tumor growth microenvironment, more predictive treatment results can be obtained not only in breast cancer patients [30,31] but in a wider range of tumor types. Because the vast majority of tumors are surrounded by adipocytes and serve as active endocrine tissue, there may be direct effects of adipocytes on tumor growth that make adipocytes as a whole, viable targets for new therapeutic strategies in cancer.
As obesity and diabetes affect an increasing number of people, it is essential to understand the mechanisms by which they contribute to the development and progression of specific cancers. Targeting cancer with specific therapies, but ignoring systemic metabolic dysfunction, can contribute to resistance to cancer therapy and treatment failure. [32-34]
Conclusion
In the studied group, the observed oxidative stress is independent of obesity and is mainly related to the presence of breast cancer. Breast cancer patients had significantly high levels of oxidation markers such as MDA and low levels of antioxidant markers, respectively thiols and total antioxidants. The results suggest an imbalance in redox homeostasis in these patients which can lead to an imbalance in pro and antiapoptotic processes, altered gene expression and mutations, all involved in the process of carcinogenesis. The study reiterates the importance of reducing oxidative stress to prevent the development and progression of many chronic diseases in which it plays an important role.
It is also known that oxygen radicals can involve in the process initiation of breast cancer and progression, depending on several genetic factors, hormonal, environmental, but also behavioral, namely nutrition, therefore. It is important to take into consideration a healthy lifestyle with an activity regular physical exercise in order to avoid any complications leading to serious damage and damaging.
Conflict of Interest
The Authors declare that they have no competing interests in relation to this study.
Author's Contributions
IP and LNG was responsible writing of the manuscript. AZ, GLS were responsible for reviewing and editing of the manuscript. DEG, IN, LSCM, AMS and MIG made substantial contributions to the conception or design of the work. RA were responsible for the critically reviewed the manuscript. All Authors read and approved the final manuscript.
REFERENCES
- https://gco.iarc.fr/causes/obesity/tools-pie
- Chan DSM, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25:1901-1914.
- Divella R, De Luca R, Abbate I, Naglieri E, Daniele A. Obesity and cancer: The role of adipose tissue and adipo-cytokines-induced chronic inflammation. J Cancer. 2016;7:2346-2359.
- Calaf GM, Urzua U, Termini L, Aguayo F. Oxidative stress in female cancers. Oncotarget. 2018;9:23824-23842.
- Henderson B, Pike M, Ross R. Epidemiology and risk factors. In: BGJ, editor. Breast Cancer: Diagnosis and Management. New York: John Wiley and Sons Ltd. 1984
- Georgescu MT, Tanase A, Dumitrache M, Ileanu B, Anghel R. Dosimetric evaluation on conventional and 3D conformal brachytherapy treatment for cervix cancer. Rom Reports Phyicss. 2017;69:1-14.
- Malins DC, Holmes EH, Polissar NL, Gunselman SJ. The etiology of breast cancer. Characteristic alteration in hydroxyl radical-induced DNA base lesions during oncogenesis with potential for evaluating incidence risk. Cancer. 1993;71:3036-3043.
- Pathak S, Wilczyński JR,Paradowska E. Factors in Oncogenesis: Viral infections in ovarian cancer. Cancers. 2020;12:561.
- Hecht F, Pessoa CF, Gentile LB, Rosenthal D, Carvalho DP, Fortunato RS. The role of oxidative stress on breast cancer development and therapy. Tumour Biol. 2016;37:4281-4291.
- Place AE, Jin Huh S, Polyak K. The microenvironment in breast cancer progression: Biology and implications for treatment. Breast Cancer Res. 2011;13:227.
- Bandini E, Rossi T, Gallerani G, Fabbri F. Adipocytes and microRNAs crosstalk: A key tile in the mosaic of breast cancer microenvironment. Cancers. 2019;11:1451.
- Carbonneau MA, Peuchant E, Sess D, Canioni P, Clerc M. Free and bound malondialdehyde measured as thiobarbituric acid adduct by HPLC in serum and plasma. Clin Chem. 1991;37:1423-1429.
- Nielsen F, Mikkelsen BB, Nielsen JB, Andersen HR, Grandjean P. Plasma malondialdehyde as biomarker for oxidative stress: Reference interval and effects of life-style factors. Clin Chem. 1997;43:1209-1214.
- Albini A. Standardization of a method of determining the thiolic-SH groups in human blood due to kinetic considerations. Boll Soc Ital Biol Sper.1980;56:1892-1898.
- Halliwell B. Antioxidant characterization. Methodology and mechanism. Biochem Pharmacol. 1995;49:1341-1348.
- Keaney JF Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, et al. Obesity and systemic oxidative stress: Clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23:434-439.
- Lee KR, Hwang IC, Han KD, Jung J, Seo MH. Waist circumference and risk of breast cancer in Korean women: A nationwide cohort study. Int J Cancer. 2018;142:1554-1559.
- Pala FS, Gürkan H. The role of free radicals in ethiopathogenesis of diseases. Adv Mol Biol. 2008;1:1-9.
- Welsch CW. Review of the effects of dietary fat on experimental mammary gland tumorigenesis: Role of lipid peroxidation. Free Radic Biol Med. 1995;18:577-773.
- Huang Z, Hankinson SE, Colditz GA, Stampfer MJ, Hunter DJ, Manson JE, et al. Dual effects of weight and weight gain on breast cancer risk. JAMA. 1997;278:1407-1141, 1997.
- Cejas P, Casado E, BeldaIC, De Castro J, Espinosa E, Redondo A, et al. Implications of oxidative stress and cell membrane lipid peroxidation in human cancer (Spain). Cancer Causes Control. 2004;15:707-719.
- Gago DM, Castelao JE, Pike MC, Sevanian A, Haile RW. Role of lipid peroxidation in the epidemiology and prevention of breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:2829-2839.
- Gnatiuc L, Alegre-Díaz J, Wade R, Ramirez-Reyes R, Tapia-Conyer R, Garcilazo-Ávila A, et al. General and abdominal adiposity and mortality in mexicocity: A prospective study of 150 000 adults. Ann Intern Med. 2019;171:397-405.
- Saintot M, Mathieu-Daude H, Astre C, Grenier J, Simony-Lafontaine J, Gerber M. Oxidant-antioxidant status in relation to survival among breast cancer patients. Int J Cancer. 2002;97:574-579.
- Eryilmaz MA, Kozanhan B, Solak I, Çetinkaya ÇD, Neselioglu S, Erel Ö. Thiol-disulfide homeostasis in breast cancer patients. J Cancer Res Ther. 2019;15:1062-1066.
- Yildirim M, Turkyilmaz E, Neselioglu S, Alisik M, Avsar AF. Dynamic thiol-disulphide status in polycystic ovary syndrome and its association with the pathogenesis of the disease. Gynecol Obstet Invest. 2017;82:54-59.
- Kemp M, Go YM, Jones DP. Nonequilibrium thermodynamics of thiol/disulfide redox systems: A perspective on redox systems biology. Free RadicBiol Med. 2008;44:921-937. 2008.
- Lin J, Cook NR, Albert C, Zaharris E, Gaziano JM, Van Denburgh M, et al. Vitamins C and E and beta carotene supplementation and cancer risk: A randomized controlled trial. J Natl Cancer Inst. 2009;101:14-23.
- Zirpoli GR, McCann SE, Sucheston-Campbell LE, Hershman DL, Ciupak G, Davis W, et al. Supplement use and chemotherapy-induced peripheral neuropathy in a cooperative group trial (S0221): The DELCaP Study. J Natl Cancer Inst. 2017;109:098.
- Bacinschi XE, Zgura A, Safta I,Anghel R. Biomolecularfactors represented by Bcl-2, p53, and tumor-infiltrating lymphocytes predict response for adjuvant anthracycline chemotherapy in patients with early triple-negative breast cancer. Cancer Manag Res. 2020;12:11965-11971.
- Botnariuc I, Ilie SM, Trifanescu OG, Bacinschi XE, Curea F, Anghel RM. Predictive circulating markers for anthracycline chemotherapy in non-metastatic breast cancer. Acta Endocrinol (Buchar). 2017;13:209-214.
- Kang C, LeRoith D, Gallagher EJ. Diabetes, Obesity, and Breast Cancer. Endocrinology. 2018;159:3801-3812.
- Zgura A, Gales L, Bratila E, Mehedintu C, Haineala B, Barac RI, et al. Variation of the T Lymphocytes according to treatment in breast cancer. Rev Chim. 2019;70:1649-1654. 2019.
- Popa-Velea O, Bacinschi X. Dynamics of anxiety in cervical cancer patients undergoing radiotherapy: Preliminary results. J Psychosom Res. 2019;121:135.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.