Hearing Pattern of a Brazilian Population Group in the State of Para
Received: 05-Jul-2022, Manuscript No. AMHSR-22-72066; Editor assigned: 07-Jul-2022, Pre QC No. AMHSR-22-72066; Reviewed: 22-Jul-2022 QC No. AMHSR-22-72066; Revised: 28-Jul-2022, Manuscript No. AMHSR-22-72066; Published: 03-Aug-2022
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Objectives: The objective of the present study was to characterize the audiological profile of a population in the Amazon, using data from the results of audiometry. Methods: The study is characterized by being a crosssectional, observational and retrospective study, with descriptive and analytical analysis. The research was carried out in a private clinic of the region. Findings: From the patients evaluated, about 57% had hearing loss. From patients with hearing loss, about 66% were female and 34% were male. A few more than 60% of patients with hearing loss were 60 years old or older. In the group of patients with hearing loss, about 75% had bilateral loss and 25% unilateral. About 60% had mild hearing loss, 30% moderate and 10% had moderately severe, severe or profound hearing loss. In the group with hearing loss, 84% had sensory loss, 7% had conductive loss and 9% had mixed loss. Conclusion: Bilateral and sensorineural hearing loss were more prevalent when compared to unilateral and conductive hearing loss. Although more hearing loss was found in elderly patients, statistical significance was noticed only by correlating mild losses with the early adulthood age group. The present study appears as a starting point for the approach of the Amazonian population by other studies.
Keywords
Hearing loss; Audiometry; Pure-Tone; Health profile
Introduction
The need to integrate and connect with the environment in which we live is in the essence of human existence. This process is complex and multifactorial, with several steps to reach the final objective of communication: the sound emission, the sound capture, the understanding of the message contained in this sound and the action taken from this coded message which can be anything from a verbal response to a physical action. Therefore, an interaction between the peripheral sound capture system is necessary, together with its interpretation, especially in the neural auditory center. Thus, there must be peripheral and central coordination so that the speech-listening binomial is at its maximum functioning stage in order to exercise this human functionality [1,2].
Hence, an alteration in this auditory detection capacity can generate great biopsychosocial impacts on human development, regardless the age group. The existence of a hearing loss in children, for example, can impact their understanding of the world and the interaction with others. It was observed that a moderate hearing deficit early in life promotes a reduction in the child's expressive vocabulary, which can be alleviated by the use of hearing aids. However, early intervention with these prostheses is of fundamental importance, due to the progressive and increasing development
– a fact that corroborates the need for prior detection of these alterations [3].
At the opposite end of the spectrum, the Age-Related Hearing Loss (ARHL) is linked to dementia, cognitive decline, and depression. In this social group, hearing loss is also associated with loneliness and social isolation which, together, increase mortality in the elderly and health expenses. However, while the ARHL is highly prevalent, this condition is mostly untreated. The correction of under detection and negligence can avoid major impacts on the quality of life of the elderly [4,5].
Therefore, it is important to carry out the screening and detection of auditory thresholds through the pure tone audiometry test and vocal audiometry. The Pure Tone Audiometry is an exam, in which auditory stimuli in different intensities and volumes are emitted to the patient to quantitatively and qualitatively diagnose the hearing loss [6]. The test starts with the emission of pure tone sounds, preferably in the ear with the best hearing, at a frequency of 1000 Hz. The “softest threshold” is the last rate at which the patient responds in at least 50% of the emitted presentations. Many studies use the average of the thresholds for frequencies of 500, 1000, 2000 and 4000 Hz as a comparative analysis [7-9].
Although there are disagreements about the use of the test as a screening exam in the asymptomatic population, there was a benefit when used in patients with complaints of hearing loss [10]. With the development of technology, it is increasingly available to access the exam, with the prospect of being consolidated, in the future, even tests via smartphones [2,11].
In addition to providing an intervention in better life conditions, the construction and analysis of an audiological profile within a population, also allows the identification of the existence of specific genetic characteristics that may be present in a population [12].
In this context, for example, the presence of alterations in the GJB2 gene is observed as one of the most common causes of hearing loss in the world, but a specific mutation (c.35delG) is more prevalent in Caucasians from Europe [13]. At the same time, more evidence of the MYO15A mutation is found in populations close to China [14].
It is important to say that are many others explanations to hearing loss, not only genetics. Loud music in earphones, diseases–like the TORCH infections – or the exposure to noise can be the reasons to problems in hearing [12].
The benefit that the observation of an audiological pattern can bring to the population is noticeable. It has become evident that hearing loss alterations are different according to localities, not only among countries, but among peoples–above all because of different genetic origins. The Amazonian population, that is rich in miscegenation between Indians and foreigners, may have different characteristics of hearing disorders compared to other parts of the world. However, there were no academic records of such analysis. In this study, therefore, there is an urgent need to discuss and analyze such inferences to revert them into benefit for the local population.
According to WHO data, the world is facing a growing prevalence of hearing loss, associated with population growth [15]. According to data from 2012, 180 million elderly people in the world were living with disabling hearing loss. More recent data from the Institution indicates an increase in this disabling deficit from 360 million people worldwide in 2008 to 466 million in 2018. It is estimated that more than 750 billion dollars are spent annually for untreated hearing loss in the world.
In this published report, the American Continent is not one of the most affected regions, however, there are no reports, especially in the Amazon, of studies analyzing this perspective. Thus, the present study aims to identify a pattern in this population, in order to verify the possibility of an eventual intervention to improve the quality of life.
The general objective is to characterize the audiological profile of a population in the Amazon Region, using data obtained from test results in a local clinic. The specific objectives are to describe the age, sex, quality and type of the most prevalent alterations among individuals with auditory system deficiency.
Methods
The present study consists of a cross-sectional and observational study, with analytical and descriptive analysis of patients who underwent the pure tone audiometry exam in a private Brazilian clinic in the city of Belém, capital of the State of Pará. All patients who underwent the exam on Mondays of three months randomly chosen in the year 2018 (February, March and April), without any specific campaign from the clinic on that day, were included in the survey. There were a total of 109 patients during the exposed period. Thus, the data was chosen and analyzed in a randomized manner.
Such selected patients were contacted to confirm their acceptance of participation in the research, by means of agreement via the Free and Informed Consent Form (FICF). The research objectives and procedures were clearly explained for all participants. Those who agreed to contribute to the study signed two copies of said term. All names were safeguarded, and the researchers accessed only the registration number in the clinic's chart, after the management’s authorization.
Patients with external ear canal occlusion or cerumen plug were excluded from the study. In addition, data from individuals with difficulties to perform the exam, either due to severe claustrophobia or anxiety, were also discarded. Minors, people with mental disorders, individuals who refused to participate in the research or patients who could not be contacted via telephone or e-mail were not included in the study sample.
The data was obtained through the use of the storage software of the specialized clinic in question, and the results of the exams were observed. The data was classified according to certain variables referring to the analysis of the audiological profile of the population studied, such as the patient's age (separated by age groups), their gender, the possible audiological alteration identified, the quality and type of this alteration.
The general objective is to characterize the audiological profile of a population in the Amazon Region, using data obtained from test results in a local clinic. The specific objectives are to describe the age, sex, quality and type of the most prevalent alterations among individuals with auditory system deficiency. of the exams were observed. The data was classified according to certain variables referring to the analysis of the audiological profile of the population studied, such as the patient's age (separated by age groups), their gender, the possible audiological alteration identified, the quality and type of this alteration.
The data was organized in Microsoft Excel 2016 program. The graphs and tables were built using tools available in Microsoft Word, Excel and Bioestat 5.5 programs. All tests were performed using the Bioestat 2008 software. The qualitative variables were described by frequencies and percentages. Confidence intervals of 95% were calculated for the proportion to infer how the prevalence behave in relation to the population from which they were obtained. The independence or association between two categorical variables was tested by the chi-square test and the significant associations were detailed by the analysis of standardized residuals, to identify the categories that contributed the most to the result. The results with p ≤ 0.05 (bilateral) were considered statistically significant.
All the subjects of the study were analyzed according to the precepts stipulated by the Declaration of Helsinki and the Nuremberg Code, respecting the Norms of Research Involving Human Beings of the National Health Council, by signing the Free and Informed Consent Form, developed by the researchers using appropriate language level to the population, an adequate prior qualification of the researchers was exercised to carry out the research. Furthermore, the study was made only after the bureaucratic authorizations of the participating centers and, obviously, after the consent of the Research Ethics Committee.
Results
In the Table 1 (Overview of patients treated at a private clinic, from February to April, 2018, Belem-Para.)
| Table 1: Overview of patients treated at a private clinic, from February to April, 2018, Belem-Para. | ||||
|---|---|---|---|---|
| Variable | Frequency | Percentage | CI95% | |
| Hearing | ||||
| Normal | 47 | 43,1 | 33,8-52,9 | |
| With Loss | 62 | 56,9 | 47,1-66,2 | |
The percentages are relative to the total number of patients evaluated (n=109) CI95%: 95% confidence interval for prevalence.
In the Table 2 (Demographic characteristics of patients with hearing loss among patients treated at a private clinic, from February to April, 2018, Belem-Para.)
| Table 2: Demographic characteristics of patients with hearing loss among patients treated at a private clinic, from February to April, 2018, Belem-Para. | |||
|---|---|---|---|
| Variable | Frequency | Percentage | |
| Sex | |||
| Male | 21 | 33,9 | |
| Female | 41 | 66,1 | |
| Age Group | |||
| 18-29 Years old | 0 | 0 | |
| 30-49 Years old | 13 | 21 | |
| 50-59 Years old | 11 | 17,7 | |
| 60-69 Years old | 19 | 30,6 | |
| 70 or More | 19 | 30,6 | |
The percentages are relative to the total number of patients with hearing loss (n=62).
In the Figure 1 (Demographic Characteristics)
The percentages are relative to the total number of patients with hearing loss (n=62).
In the Table 3 (Type and quality of hearing disorders in patients treated at a private clinic, from February to April, 2018, Belem-Para.)
| Table 3: Type and quality of hearing disorders in patients treated at a private clinic, from February to April, 2018, Belem-Para. | ||||
|---|---|---|---|---|
| Variable | Frequency | Percentage | CI95% | |
| Disorder Type | ||||
| Bilateral | 47 | 75,8 | 63,0-85,4 | |
| Unilateral | 15 | 24,2 | 14,6-37,0 | |
| Disorder Quality | ||||
| Mild | 37 | 59,7 | 46,5-71,7 | |
| Moderate | 20 | 32,3 | 21,3-45,5 | |
| Moderately Severe | 3 | 4,8 | 1,3-14,4 | |
| Severe | 1 | 1,6 | 0,1-9,8 | |
| Profound | 1 | 1,6 | 0,1-9,8 | |
The percentages are relative to the total number of patients with hearing loss (n=62). 95%CI: 95% confidence interval for prevalence.
In the Table 4(Type of hearing loss in patients treated at a private clinic, from February to April 2018, Belem-Para.)
| Table 4: Type of hearing loss in patients treated at a private clinic, from February to April 2018, Belem-Para. | ||||
|---|---|---|---|---|
| Variable | Frequency | Percentage | CI95% | |
| Type of Hearing Loss | ||||
| Mixed | 6 | 9,7 | 4,0-20,5 | |
| Sensorineural (Neurosensory) | 52 | 83,9 | 71,9-91,6 | |
| Conductive | 4 | 6,5 | 2,1-16,5 | |
The percentages are relative to the total number of patients with hearing loss (n=62). 95%CI: 95% confidence interval for prevalence.
In the Table 5 (Relationship between hearing disorders and age of patients treated at a private clinic, from February to April, 2018, Belem-Para.)
| Table 5: Relationship between hearing disorders and age of patients treated at a private clinic, from February to April, 2018, Belem-Para. | |||||||
|---|---|---|---|---|---|---|---|
| Variable | Mild (n=37) | Moderate (n=20) | M Severe (n=3) | Severe (n=1) | Profound (n=1) | p- value | |
| Age Group | 0,115 | ||||||
| 30-49 Years old | 12 (32,4) | 0 (0,0) | 0 (0,0) | 0 (0,0) | 1 (100,0) | ||
| 50-59 Years old | 6 (16,2) | 4 (20,0) | 1 (33,3) | 0 (0,0) | 0 (0,0) | ||
| 60-69 Years old | 11 (29,7) | 8 (40,0) | 0 (0,0) | 0 (0,0) | 0 (0,0) | ||
| 70 or More | 8 (21,6) | 8 (40,0) | 2 (66,7) | 1 (100,0) | 0 (0,0) | ||
Categorical variables are displayed as n (%). The percentages are relative to the total of each column. Chi-square was used.
In the Table 6 (Relationship between hearing disorders and age, considering the mild, moderate and severe state of patients treated at a private clinic, from February to April, 2018, Belem-Para.)
| Table 6: Relationship between hearing disorders and age, considering the mild, moderate and severe state of patients treated at a private clinic, from February to April, 2018, Belem-Para. | ||||
|---|---|---|---|---|
| Variable | Mild (n=37) | Moderate (n=20) | M Severe to Profound (n=5) | p-value |
| Age Group | 0,069 | |||
| 30-49 Years old | 12 (32,4) | 0 (0,0) | 1 (20,0) | |
| 50-59 Years old | 6 (16,2) | 4 (20,0) | 1 (20,0) | |
| 60-69 Years old | 11 (29,7) | 8 (40,0) | 0 (0,0) | |
| 70 or more | 8 (21,6) | 8 (40,0) | 3 (60,0) | |
Categorical variables are displayed as n (%). The percentages are relative to the total of each column. Chi-square was used.
In the Table 7 (Relationship between hearing alterations and age, comparing the mild state with the moderate to severe state of patients treated at a private clinic, from February to April, 2018, Belem-Para.)
| Table 7: Relationship between hearing alterations and age, comparing the mild state with the moderate to severe state of patients treated at a private clinic, from February to April, 2018, Belem-Para. | ||||
|---|---|---|---|---|
| Variable | Mild (n=37) | Moderate to Profound (n=25) | -value | |
| Age Group | 0,040 | |||
| 30-49 Years old | 12 (32,4)† | 1 (4,0)* | ||
| 50-59 Years old | 6 (16,2) | 5 (20,0) | ||
| 60-69 Years old | 11 (29,7) | 8 (32,0) | ||
| 70 or more | 8 (21,6) | 11 (44,0) | ||
Categorical variables are displayed as n (%). The percentages are relative to the total of each column. Chi-square was used.
*: this frequency was lower than what would be expected by chance.
†: this frequency was higher than expected.
In the Figure 2 (Relationship between hearing alterations and age, comparing the mild state with the moderate to severe state.)
The chi-square was used: p=0.040.
*: this frequency was lower than what would be expected by chance.
†: this frequency was higher than expected.
Discussion
According to the World Health Organization, hearing losses can be classified in different degrees, through the correspondence of the audiometric average values of the four frequency groups mentioned before (500, 1000, 2000 and 4000 Hz) [16]. A grade of 0 indicates no impairment, with hearing loss less than 20 dB in the best ear. In grade 1, the frequency varies between 20 and 34 dB, indicating a mild hearing loss. Thereafter, the grades 2, 3, 4, 5 and 6 indicate disabling hearing loss in adults. The grade 2 indicates moderate hearing loss (best ear picks up between 35 and 49 dB); the grade 3 indicates moderately severe hearing loss (between 51 and 64 dB); the grade 4 indicates severe hearing loss (between 65 and 79 dB); the grade 5 indicates profound hearing loss (between 80 and 94 dB) and, finally, the grade 6 indicates complete or total deafness including alteration of at least 95 dB in the best ear. The World Health Organization indicates benefit with the use of hearing aids from grade 2 onwards.
In addition to this quantitative classification, a guide prepared in collaboration with the Brazilian Society of Speech Therapy and Brazilian Academy of Audiology brings the classic classification by Lloyd and Kaplan from 1978, in which six degrees are described: normal hearing (≤ 25 dB), mild hearing loss (26-40 dB), moderate hearing loss (41-55 dB), moderately severe hearing loss (56 dB-70 dB), severe hearing loss (71 dB-90 dB) and deep hearing loss (≥ 91 dB) – always considering the values in the best ear [17].
In addition to this quantitative change, a qualitative distinction can be made in hearing loss: Sensorineural, conductive or mixed. The conductive alterations may result from alterations in the middle ear, external auditory canal or tympanic membrane, due to different etiologies, such as otosclerosis, ear canal obstruction by cerumen, tympanic perforation or otitis media with effusion. On the other hand, neurosensory alterations can range from cerebellar tumors, sudden losses and Meniere's disease, to presbycusis and exposure to ototoxins. The mixed type combines changes in mechanical sound conduction with neuroelectric changes [12,18]. Thus, the analysis of the epidemiological profile of hearing disorders in the studied Amazonian population begins with the highest prevalence of hearing loss in more than half of the 109 patients evaluated, corresponding to a percentage of 56.9%-Table 1. This is explained by the absence of screening method for hearing loss in adults, not only by the Brazilian Health Ministry, but also by the USPSTF (U.S. Preventive Services Task Force) – which published in 2021 the persistence of insufficient data to validate a possible screening [19]. In other words, the search for exams is mainly based on clinical complaints, justifying the result that was found.
According to the World Health Organization [16], hearing losses can be classified in different degrees, through the correspondence of the audiometric average values of the four frequency groups mentioned before (500, 1000, 2000 and 4000 Hz). A grade of 0 indicates no impairment, with hearing loss less than 20 dB in the best ear. In grade 1, the frequency varies between 20 and 34 dB, indicating a mild hearing loss. Thereafter, the grades 2, 3, 4, 5 and 6 indicate disabling hearing loss in adults. The grade 2 indicates moderate hearing loss (best ear picks up between 35 and 49 dB); the grade 3 indicates moderately severe hearing loss (between 51 and 64 dB); the grade 4 indicates severe hearing loss (between 65 and 79 dB); the grade 5 indicates profound hearing loss (between 80 and 94 dB) and, finally, the grade 6 indicates complete or total deafness including alteration of at least 95 dB in the best ear. The World Health Organization indicates benefit with the use of hearing aids from grade 2 onwards.
In addition to this quantitative classification, a guide prepared in collaboration with the Brazilian Society of Speech Therapy and Brazilian Academy of Audiology brings the classic classification by Lloyd and Kaplan from 1978, in which six degrees are described: normal hearing (≤ 25 dB), mild hearing loss (26-40 dB), moderate hearing loss (41-55 dB), moderately severe hearing loss (56-70 dB), severe hearing loss (71-90 dB) and deep hearing loss (≥ 91 dB) – always considering the values in the best ear [17].
In addition to this quantitative change, a qualitative distinction can be made in hearing loss: sensorineural, conductive or mixed. The conductive alterations may result from alterations in the middle ear, external auditory canal or tympanic membrane, due to different etiologies, such as otosclerosis, ear canal obstruction by cerumen, tympanic
According to the WHO Report earlier mentioned in this study 11, more than 460 million people worldwide have disabling hearing loss. According to the research findings, in Table 2, the female sex covered most of the patients with losses, with a number corresponding to 66% of the patients. A Swedish study also found a greater number of women in its sample, however there was no statistical significance [20]. However, considering Acquired Sensory Hearing Loss–the most common type, which etiology is degeneration associated with noise exposure, increasing age, ototoxicity and other entities–there is a prevalence of males [21]. One of the justifications found in the literature suggests not only a higher prevalence, but also the occurrence of early and more severe forms in males. The labor difference found in the professional practice between men and women would explain this fact [22]. Hence, men are more exposed to noise.
Nevertheless, when exposed to the same level of intensity and frequency of noise, men and women of similar ages present significant differences in hearing preservation. In the studies analyzed, women showed better hearing thresholds after exposure to noise, while men had a higher risk of developing high-frequency hearing loss [23]. The mechanisms are not fully understood, but it is possible to agree on an association between estrogen levels and a better prognosis related to hearing, which would be one of the justifications for the asymmetry noted between men and women, not ignoring the multifactorial nature of the differences [24]. When comparing the effectiveness of rehabilitation of men and women with severe to profound loss, no gender difference was found in the success of therapy [25].The analysis of Figure 1 allows us to infer that 61.2% of patients with hearing loss are 60 years old or older than that, indicating a higher prevalence of alterations in the elderly. The most prevalent sensory deficit in the elderly, according to Bowl&Dawson, is age-related hearing loss [26]. Although the understanding of genes is increasingly widespread, age-related hearing loss still has strong possibilities of genetic influence that confer a greater predisposition to it, while this has not been proven so far.
A study with autopsies of human inner ears made it possible to compare their composition with recent audiometries of these patients. It was noticed that the main mechanism of age-related loss would be associated with damage to the interior of these sensory cells, rather than the loss of cellular capacity itself. Thus, lifetime acoustic overexposure is a major risk factor for this condition [27].
Furthermore, there is an urgent need to contest one of the main forms of hearing damage: oxidative stress. However, this mechanism is also one of the most difficult to intervene, since it is a natural and multifactorial process of human aging. Thus, the study of the Nuclear factor erythroid-2-related factor 2 (Nrf2) as a reducer of cell damage may prove to be a great ally in drugs that target hearing protection, if further studies corroborate greater safety and pharmacological efficacy [28].
For a better analysis of the proportions, 95% confidence intervals were calculated for the prevalence described in Table 3. A narrow interval indicates greater certainty related to the prevalence in the studied population. Therefore, almost 60% of the results were obtained indicating mild alteration (IC 46.5–71.7).
Also according to Table 3, most patients (75.8%) have bilateral alterations, while the minority have unilateral alterations. The most recent WHO classification (WHO, 2021) indicates that there is unilateral hearing loss when the hearing threshold is less than 20 dB in the best ear and 35 dB or more in the worst ear. A study carried out in the United States found a prevalence of 7.2% of unilateral hearing loss in the American population, corroborating the finding that unilateral hearing loss is less prevalent compared to other types of hearing loss [29].
The main reason that bilateral hearing loss is predominant is probably the fact that presbycusis is mainly associated with bilateral hearing loss, considering that the population found was mostly elderly [30]. This fact is corroborated by Table 4, in which 83.9% of patients with hearing alterations have sensorineural hearing loss.
The Tables 5, 6 and 7 demonstrate the association between the quality of hearing loss and the age range of the patients. Table 5 shows the association between age group and level of hearing loss. In subjects with mild loss, 32.4% of patients were between 30 and 49 years old, while 29.7% were between 60 and 69 years old. In the moderate loss group, 80% were 60 years old or older. In subjects with moderately severe and severe loss, the majority were also elderly. However, these observed differences did not reach statistical significance (p=0.115).
A Danish study described an association of diagnosed hearing loss, whether in early adulthood, late adulthood or the elderly phase, with a higher risk of dementia in the population with hearing deficit, regardless of the cognitive ability or level of education of the patient [31]. This isolated risk factor demands monitoring of these individuals, especially with frequent cognitive analysis.
In Table 6, the groups with mild and moderate alterations and a single group formed by patients with moderately severe to profound loss are compared. Also in this case, the differences remain non-significant (p=0.069).
Table 7 compares the group with mild alteration and a single group formed by patients with moderate to profound loss. In this case, age and level of loss were significantly associated (p=0.040): from the 37 individuals with mild loss, 12 (32.4%) were between 30 and 49 years old, in a greater proportion than expected, and, in the moderate to profound group, only one was in this age group. In other words, mild hearing loss was associated with a lower age group–as visually demonstrated in Figure 2.
Osler et al [31]. also found a relationship between hearing loss in young adulthood and decreased cognitive capacity in this group, a fact that encourages the importance of mild hearing loss group monitoring due to possible future changes – reinforcing the necessity of early screening methods still under debate. A relatively simple management in these patients could avoid major future consequences.
Therefore, the need for auditory rehabilitation in patients with some type of loss is reinforced, either through relatively simple hearing aids to cochlear implants–both impacting the patient's life quality. However, the use of implant devices at the bone level is shown to be superior in terms of improvements for the individual. In a German study, a significant change in gait and dementia assessment tests was observed in pre-implant patients compared to the same patients 6 months after the procedure–a fact that gives hope even in individuals with more severe loss [32,33].
Another fundamental factor to be analyzed in an audiological profile scenario is the genetic content of the population studied. It is known, for example, that alterations in the GJB2 and SLC26A4 genes are associated with hearing loss in the Chinese population [34]. However, in the Egyptian population, the p.Gly12Valfs*2 mutation is the most characteristic [35]. It is still possible to describe distinct genes in hearing loss from other populations, such as the MYO15A and OTOG genes in the Israeli population [36]. Thus, the alterations found in the cut of the Amazonian population, rooted in Indian origin, may also be the result of the specific genetic content of that region.
Conclusion
It were found more abnormal exams than the exams within the normal range. Most hearing alterations were found in women. In addition, bilateral and sensorineural losses were found in greater numbers. And, although there was a higher prevalence of hearing loss in patients over 60 years of age, statistical significance was found only when there was a correlation of mild losses with a lower age group. Finally, the analysis of this information appears as a starting point for more studies involving this population, in order to ponder the needs of attention to hearing loss in the Amazon.
Conflicts of Interest
The authors declare that the research was conducted without conflict of interest or any public or private financial support.
References
- Qian M, Wang Q, Yang L, Wang Z, Hu D, Li B, et al. The effects of aging on peripheral and central auditory function in adults with normal hearing. Am J Transl Res. 2021;13:549-64.
[Crossref], [Google Scholar], [Indexing]
- Masalski M, Grysiński T, Kręcicki T. Hearing tests based on biologically calibrated mobile devices: Comparison with pure-tone audiometry. JMIR Mhealth Uhealth. 2018 ;6:e10.
[Crossref], [Google Scholar], [Indexing]
- Persson A, Marklund U, Lohmander A, Flynn T. Expressive vocabulary development in children with moderate hearing loss-the impact of auditory variables and early consonant production. Clin Linguist Phon. 2022;36:547-64.
[Crossref], [Google Scholar], [Indexing]
- Sharma RK, Chern A, Golub JS. Age-related hearing loss and the development of cognitive impairment and late-life depression: A scoping overview. Semin Hear. 2021;42:10-25.
[Crossref], [Google Scholar], [Indexing]
- Shukla A, Harper M, Pedersen E, Goman A, Suen JJ, Price C, et al. Hearing loss, loneliness, and social isolation: A systematic review. Otolaryngol Head Neck Surg. 2020;162:622-33.
[Crossref], [Google Scholar], [Indexing]
- Michels TC, Duffy MT, Rogers DJ. Hearing loss in adults: Differential diagnosis and treatment. Am Fam Physician. 2019;100:98-108.
[Crossref], [Google Scholar], [Indexing]
- Oosterloo BC, Homans NC, Baatenburg de Jong RJ, Ikram MA, Nagtegaal AP, Goedegebure A. Assessing hearing loss in older adults with a single question and person characteristics; comparison with pure tone audiometry in the rotterdam study. PLoS One. 2020;15:e0228349.
[Crossref], [Google Scholar], [Indexing]
- Carl AC, Cornejo J. Audiology pure tone evaluation. 2022. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022.
- Kwok SS, Nguyen XT, Wu DD, Mudar RA, Llano DA. Pure tone audiometry and hearing loss in alzheimer's disease: A meta-analysis. Front Psychol. 2022;12:788045.
[Crossref], [Google Scholar], [Indexing]
- Louw C, Swanepoel W, Eikelboom RH. Self-reported hearing loss and pure tone audiometry for screening in primary health care clinics. J Prim Care Community Health. 2018;9:2150132718803156.
[Crossref], [Google Scholar], [Indexing]
- Sandstrom J, Swanepoel D, Laurent C, Umefjord G, Lundberg T. Accuracy and reliability of smartphone self-test audiometry in community clinics in low income settings: A comparative study. Ann Otol Rhinol Laryngol. 2020;129:578-84.
[Crossref], [Google Scholar], [Indexing]
- Sheffield AM, Smith RJH. The epidemiology of deafness. Cold Spring Harb Perspect Med. 2019
- Azadegan-Dehkordi F, Ahmadi R, Koohiyan M, Hashemzadeh-Chaleshtori M. Update of spectrum c.35delG and c.-23+1G>A mutations on the GJB2 gene in individuals with autosomal recessive nonsyndromic hearing loss. Ann Hum Genet. 2019;83:1-10.
[Crossref], [Google Scholar], [Indexing]
- Zhang J, Guan J, Wang H, Yin L, Wang D, Zhao L, et al. Genotype-phenotype correlation analysis of MYO15A variants in autosomal recessive non-syndromic hearing loss. BMC Med Genet.;20:60.
[Crossref], [Google Scholar], [Indexing]
- WHO. Addressing the rising prevalence of hearing loss. Geneva: World Health Organization; 2018.
- https://www.who.int/publications/i/item/world-report-on-hearing.
- https://audiology-web.s3.amazonaws.com/migrated/201208_AudGuideAssessHear_youth.pdf_5399751b249593.36017703.pdf
- Young YH. Contemporary review of the causes and differential diagnosis of sudden sensorineural hearing loss. Int J Audiol. 2020 ;59:243-53.
[Crossref], [Google Scholar], [Indexing]
- Krist AH, Davidson KW, Mangione CM, Cabana M, Caughey AB, et al. Screening for hearing loss in older adults: Us preventive services task force recommendation statement. JAMA. 2021;3 25:1196-1201.
[Crossref], [Google Scholar], [Indexing]
- Dahlin Redfors Y, Jönsson R, Finizia C. A validation study of the Swedish version of the Glasgow hearing aid benefit profile evaluated in otosclerosis subjects. Laryngoscope Investig Otolaryngol. 2022 ;7:807-15.
[Crossref], [Google Scholar], [Indexing]
- Ren Y, Landegger LD, Stankovic KM. Gene therapy for human sensorineural hearing loss. Front Cell Neurosci. 2019;13:323.
[Crossref], [Google Scholar], [Indexing]
- Nolan LS. Age-related hearing loss: Why we need to think about sex as a biological variable. J Neurosci Res. 2020;98:1705-20.
[Crossref], [Google Scholar], [Indexing]
- Lien KH, Yang CH. Sex differences in the triad of acquired sensorineural hearing loss. Int J Mol Sci. 2021;22:8111.
[Crossref], [Google Scholar], [Indexing]
- Shuster BZ, Depireux DA, Mong JA, Hertzano R. Sex differences in hearing: Probing the role of estrogen signaling. J Acoust Soc Am. 2019;145:3656.
[Crossref], [Google Scholar], [Indexing]
- Turunen-Taheri S, Carlsson PI, Johnson AC, Hellström S. Severe-to-profound hearing impairment: Demographic data, gender differences and benefits of audiological rehabilitation. Disabil Rehabil. 2019 ;41:2766-74.
[Crossref], [Google Scholar], [Indexing]
- Bowl MR, Dawson SJ. Age-related hearing loss. Cold Spring Harb Perspect Med. 2019;9:a033217.
[Crossref], [Google Scholar], [Indexing]
- Wu PZ, O'Malley JT, de Gruttola V, Liberman MC. Age-related hearing loss is dominated by damage to inner ear sensory cells, not the cellular battery that powers them. J Neurosci. 2020 Aug 12;40:6357-66.
[Crossref], [Google Scholar], [Indexing]
- Li D, Zhao H, Cui ZK, Tian G. The Role of Nrf2 in Hearing Loss. Front Pharmacol. 2021 ;12:620921.
[Crossref], [Google Scholar], [Indexing]
- Golub JS, Lin FR, Lustig LR, Lalwani AK. Prevalence of adult unilateral hearing loss and hearing aid use in the United States. Laryngoscope. 2018;128:1681-86.
[Crossref], [Google Scholar], [Indexing]
- Löhler J, Cebulla M, Shehata-Dieler W, Volkenstein S, Völter C, Walther LE. Hearing impairment in old age. Dtsch Arztebl Int. 2019;116:301-10.
[Crossref], [Google Scholar], [Indexing]
- Osler M, Christensen GT, Mortensen EL, Christensen K, Garde E, Rozing MP. Hearing loss, cognitive ability, and dementia in men age 19-78 years. Eur J Epidemiol. 2019;34:125-30.
[Crossref], [Google Scholar], [Indexing]
- Issing C, Baumann U, Pantel J, Stöver T. Impact of hearing rehabilitation using cochlear implants on cognitive function in older patients. Otol Neurotol. 2021;42:1136-41.
[Crossref], [Google Scholar], [Indexing]
- Brodie A, Smith B, Ray J. The impact of rehabilitation on quality of life after hearing loss: A systematic review. Eur Arch Otorhinolaryngol. 2018;275:2435-40.
[Crossref], [Google Scholar], [Indexing]
- Zhu Y, Hu L, Yang L, Wang L, Lu Y, Dong X, et al. Association between expanded genomic sequencing combined with hearing screening and detection of hearing loss among newborns in a neonatal intensive care unit. JAMA Netw Open. 2022;5:e2220986.
[Crossref], [Google Scholar], [Indexing]
- Gibriel AA, Abou-Elew MH, Masmoudi S. Analysis of p.Gly12Valfs*2, p.Trp24* and p.Trp77Arg mutations in GJB2 and p.Arg81Gln variant in LRTOMT among non syndromic hearing loss Egyptian patients: implications for genetic diagnosis. Molecular Biology Reports. 2019;46:2139–45.
[Crossref], [Google Scholar], [Indexing]
- Danial-Farran N, Brownstein Z, Gulsuner S, Tammer L, Khayat M, Aleme O, et al. Genetics of hearing loss in the Arab population of Northern Israel. Eur J Hum Genet. 2018;26:1840-47.
[Crossref], [Google Scholar], [Indexing]

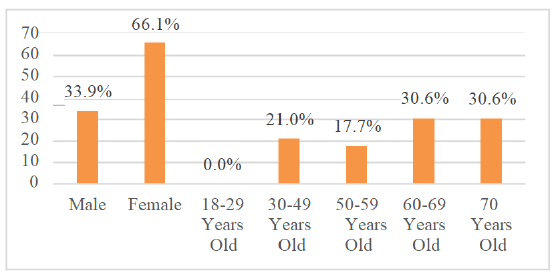
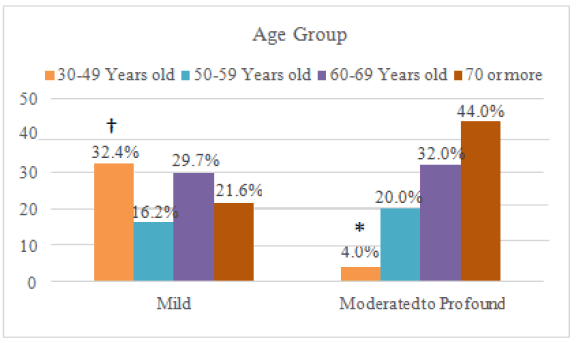



 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.