Identification of Insulin Sensitizing Activity of Gymnema sylvestre in Dexamethasone Induced Insulin Resistance in Rats
2 Department of Pharmacology, Al-Ameen Medical College, Vijayapura, Karnataka, India, Email: hems286@gmail.com
Citation: Hemanth Kumar V, et al. Identification of Insulin Sensitizing Activity of Gymnema sylvestre in Dexamethasone Induced Insulin Resistance in Rats. Ann Med Health Sci Res. 2017; 7:18-22
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: Insulin resistance plays a key role in the onset of several metabolic disorders. Aim and Objectives: To determine the insulin sensitizing property of aqueous extract of Gymnema sylvestre leaves on dexamethasone induced insulin resistance in rats. Materials and Methods: Animals were divided into five groups (n=6) and the study was conducted for a period of 12 days. Insulin resistance was induced with dexamethasone (Dex) (4 mg/kg) intraperitoneal (i.p) injection. Control (Group I) and Dexamethasone group (Group II) received gum acacia orally (p.o) for 12 days. The test groups (Group III and Group IV) received Gymnema sylvestre leaves aqueous extract (1 and 2 gm/kg/p.o. respectively) and standard drug group (Group V) received rosiglitazone (8 mg/kg/p.o) for 12 days. All the groups except control group received Dex (4 mg/kg/i.p.) from 7th day to 12th day. Insulin resistance was measured by using serum glucose, insulin levels and by performing intraperitoneal glucose tolerance test (IPGTT). The degree of Insulin resistance was calculated by using indices i.e. Homeostasis model assessment for insulin resistance (HOMA-IR), insulin sensitivity (HOMA-IS) and fasting glucose to insulin ratio (FGIR). Values were analyzed by one way analysis of variance (ANOVA) followed by Scheffe’s multiple comparison posttest. Results: Dex significantly raised serum glucose and insulin levels in comparison to control group. It induced glucose intolerance and insulin resistance confirmed by HOMA-IR, IS and FGIR. Treatment with Gymnema sylvestre extract significantly prevented all the changes induced by Dex. Conclusion: To conclude, Dex has induced insulin resistance and these abnormalities associated with IR are partially prevented with pre administration of Gymnema sylvestre.
Keywords
Gymnema sylvestre; Dexamethasone; Insulin resistance
Introduction
Stress constitutes an independent risk factor for development of several metabolic disorders. Stress of any origin either physiological or economic or catabolic may alters the metabolic pathways. Stress has become an integral part of our day to day life. [1]. Stress stimulates Hypothalamic-pituitary adrenal axis (HPA-axis) and causes the release of catabolic hormones such as glucocorticoids and also alters the functioning of inflammatory cytokines. [2].
Glucocorticoids play a vital role in several metabolic pathways. They are helpful in several clinical applications. However, excess glucocorticoids of any origin either endogenous or exogenous, leads to metabolic disorders. They increase blood glucose, insulin levels and alter lipid metabolism. These further results in a state of insulin resistance. [3].
Insulin resistance condition usually associated with excess release of insulin to lower plasma glucose levels. This hyperinsulinemia accounts for the peripheral reduction of insulin sensitivity. [4]. It is associated with various conditions such as type 2 diabetes, obesity, septicemia, poly cystic ovary syndrome and excess glucocorticoids. [5].
Dexamethasone (Dex) is a synthetic glucocorticoid. Dex, due to its anti-inflammatory and immunosuppressant properties has got wide therapeutic applications. But, the major drawback with this, it may cause glucose intolerance and reduce insulin sensitivity in some vulnerable patients, depending upon the dose and frequency of administration which can cause diabetes mellitus. [6]. Apart from its clinical uses, it is widely used in research for induction of insulin resistance in animals and human beings. [7-10].
Insulin resistance can be assessed by using fasting insulin levels. But, to obtain a more accurate idea, euglycemic hyperinsulinemic clamp technique was employed as it is considered as the gold standard method to measure insulin resistance. [11]. However, the use of this method was discouraged due to tedious work and restricted to small scale of samples. To overcome this, other methods were developed. Among them, Homeostatic assessment method (HOMA) was developed by Mathews et al., which is a calculative method based on fasting glucose and insulin levels. Later on, several other indices were developed like Quantitative insulin sensitivity check index (QUICKI), Fasting glucose to insulin ratio (FGIR) and Mastuda index etc. [11].
Gymnema sylvestre (G. sylvestre) is commonly known as Gurmar, it belongs to family Asclepediace. The leaves of this plant are being helpful in the treatment of several clinical conditions such as diabetes, obesity and certain microbial infections. [12]. Several mechanisms were postulated for its blood glucose lowering property. [13]. But, there was scarce of data on insulin sensitizing property of this plant. It was also not clear that, whether this plant will be helpful in prevention of glucocorticoid induced insulin resistance. Hence, the presented study was aimed to explore the preventive effect of G. sylvestre leaf extract in Dex induced insulin resistance.
Materials and Methods
Animals
Study was conducted on male albino Wistar rats around 6 months of age and an average weight of 230-280gms. Prior to study Institutional animal ethical committee clearance (IAEC) was obtained [KSHEMA/AEC/30/2011].. They were maintained as per guidelines of the committee for the purpose of control and supervision on experimental animals (CPCSEA). Animals were housed in polypropylene cages (UN shah Manufacturers, Mumbai, India), with 12 hrs light and 12 hrs dark cycles and under temperature of (23±2)0C. They were fed with rat pellets (Hindustan Lever Ltd, Mumbai, India) and water at ad libitium.
Drugs and chemicals
Dex was procured from Zydus pharmaceuticals, Mumbai, India. Purified sample of rosiglitazone was obtained from sigma chemicals (R2408) (Rajendra traders, Hubli, Karnataka, India). Ketamine (100 mg/0.01%/2 ml) injection was procured from Neon Laboratories, Mumbai, India.
Reagents and kits
Serum glucose was measured by using commercially available kit (Erba Mannheim, Transasia Biomedicals, Cat. No. XSYS0012). Serum insulin levels were measured by using Ultra-sensitive rat insulin enzyme linked immunosorbent assay (ELISA) kit, obtained from Gen X Bio Health Science Private Limited (Cat. No. 90060) (Provided by Crystal Chem, New Delhi).
Plant material collection and aqueous extract preparation
The leaves of the G. sylvestre plant were obtained from Vijayapura, Karnataka, India. The aqueous extract was prepared by cold percolation method. Dried leaves of G. sylvestre were taken in a round bottom flask. To that distilled water was added and kept for 24hours. Then it was filtered by distillation process and the extract was concentrated. The remaining solvents were removed by evaporating on a water bath. The extract was completely dried under vacuum. [14].
Phytochemical analysis
The dried aqueous extract was subjected to preliminary phytochemical analysis for identification of phytochemical constituents. [15-17].
Study design
In the present study animals were divided into five groups (n=6). The dose of Dex and Rosiglitazone were selected from previous studies. [18,19]. Pilot study (n=2) was conducted initially with graded doses of G. sylvestre aqueous extracts to obtain the doses. Respective doses of G. sylvestre extract and rosiglitazone were dissolved in (2%) gum acacia and they were administered to their respective groups through oral gavage. The current study was conducted for a period of 12 days. The grouping and treatment procedure as follows:
• Group I: The control group received 2 % gum acacia orally (p.o) for 12 days.
• Group II: The Dex group received, 2% gum acacia orally for 12 days and Dex (4 mg/kg/i.p.) from 7th day to 12th day.
• Group III: The test group received G. sylvestre (2 gm/kg/p.o) extract for 12 days and Dex (4 mg/kg/i.p) from 7th day to 12th day.
• Group IV: The test group received G. sylvestre (4 gm/kg/p.o) extract for 12 days and Dex (4 mg/kg/i.p) from 7th day to 12th day.
• Group V: The standard group received rosiglitazone (8 mg/ kg/p.o) for 12 days and Dex (4 mg/kg/i.p) from 7th day to 12th day.
Food was withheld overnight before the last day i.e., on 11th day. On 12th day, the fasting blood samples were collected through retro orbital sinus puncture under ketamine (50 mg/kg/i.p.) anesthesia for biochemical estimations. Then, intra peritoneal glucose tolerance test (IPGTT) was performed by administering glucose (20%) (2 gm/kg/i.p) load. [20]. Around 2 ml of blood was collected at each interval i.e., 30, 60 and 120 min.
Biochemical estimation
The collected blood samples were centrifuged at 2000 RPM for 20 minutes. Serum was separated and glucose levels were estimated by glucose oxidase peroxidase method (GOD-POD) using an automated analyzer. [17]. Insulin levels were measured by ELISA method using an ELISA reader. [19].
Calculation of insulin sensitivity indices
The following formulas were employed for estimating insulin resistance
Homeostasis model assessment for insulin resistance (IR)
(HOMA-IR) = [Fasting insulin × fasting glucose]/405 [21].
Homeostasis model assessment for insulin sensitivity (IS)
(HOMA-IS) = 10000/ [fasting insulin × fasting glucose] [21].
Fasting glucose to insulin ratio (FGIR) = Fasting glucose/ Fasting insulin [11].
Statistical analysis
The data was represented as Mean±SEM. Statistical analysis was performed using one way Analysis of Variance (ANOVA) followed by Scheffe multiple comparison test, using Statistical Package for Social Studies (SPSS, 20.0). The minimum level of significance was set at p<0.05.
Results
Phytochemical analysis
The preliminary phytochemical analysis of plant extract showed the presence of phytochemical constituents such as phenols, glycosides, flavonoids, carbohydrates, tannins and alkaloids.
On serum glucose and Insulin levels
Administration of Dex (4 mg/kg/i.p.) significantly raised serum glucose (mg/dL) and insulin (μU/mL) levels in comparison with the control group (p<0.01).
From the pilot study, it was observed that the low doses of G.sylvestre (500 mg and 1 gm/kg/p.o.) were insufficient to prevent the Dex induced raise in glucose and insulin levels [Table 1]. Pretreatment with high doses of G. sylvestre aqueous extract (2 and 4 gm/kg/p.o) significantly prevented the rise in serum glucose and insulin levels in comparison to Dex treated group [Table 2] (p<0.05).
| Groups | Fasting glucose (mg/dL) | Fasting insulin (μU/mL) |
|---|---|---|
| G. sylvestre (500 mg/kg) | 185.78 ± 3.57 | 236.4 ± 10.8 |
| G. sylvestre (1 gm/kg) | 141.28 ± 1.39 | 190.8.4 ± 18 |
| G. sylvestre (2 gm/kg) | 119.53 ± 1.69 | 84 ± 9.6 |
| G. sylvestre (4 gm/kg) | 106.84 Â ± 2.1 | 97.2 ± 13.2 |
Values were expressed in Mean ± SEM
Table 1: Pilot study (n=2) results of G. sylvestre aqueous extract.
| Groups | Fasting glucose (mg/dL) | Fasting insulin (μU/mL) | HOMA- IR | HOMA– IS | FGIR |
|---|---|---|---|---|---|
| Group I | 98.61 ± 0.87 | 74.4 ± 1.63 | 18.13 ± 0.53 | 1.36 ± 0.04 | 1.32 ± 0.02 |
| Group II | 192.83 ± 1.87* | 329.3 ± 3.93* | 156.73 ± 2.27* | 0.15 ± 0.02* | 0.58 ± 0.01* |
| Group III | 117.83 ± 1.93Â¥ †| 137.6 ± 10.49Â¥ | 39.98 ± 2.98Â¥ †| 0.63 ± 0.04Â¥ †| 0.88 ± 0.07Â¥ |
| Group IV | 113.96  ± 1.94Â¥ | 118 ± 9.30 Â¥ | 33.12 ± 2.50Â¥ | 0.76 ± 0.04Â¥ | 0.99 ± 0.08Â¥ |
| Group V | 108.50 ± 2.14Â¥ | 107.2 ± 6.09Â¥ | 28.74 ± 1.72Â¥ | 0.87 ± 0.06Â¥ | 1.02 ± 0.06Â¥ |
Values were expressed in Mean ± SEM, *p<0.01 vs. Group I, Â¥p<0.05 vs. Group II, †p<0.05 vs. Group V (HOMA-IR- Homeostasis model assessment for insulin resistance, HOMA-IS- Homeostasis model assessment for insulin sensitivity, FGIR - Fasting glucose to insulin ratio)
Table 2 : Insulin sensitizing activity of G. sylvestre aqueous extract.
IPGTT values
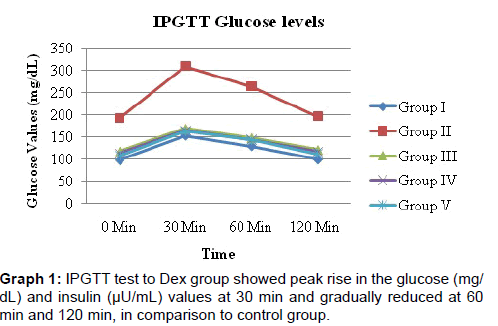
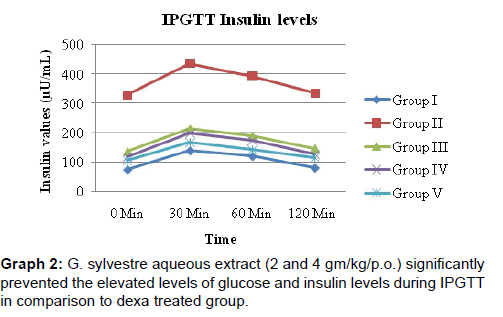
IPGTT test to Dex group showed peak rise in the glucose (mg/ dL) and insulin (μU/mL) values at 30min and gradually reduced at 60 min and 120 min, in comparison to control group [Graphs 1 and 2].
G. sylvestre aqueous extract (2 and 4 gm/kg/p.o.) significantly prevented the elevated levels of glucose and insulin levels during IPGTT in comparison to dexa treated group [Graphs 1 and 2]. Rosiglitazone also prevented the rise in fasting glucose, insulin values and rise during IPGTT [Table 2] [Graphs 1 and 2].
Insulin resistance indices
In comparison to control group Dex (4 mg/kg/i.p.) significantly induced insulin resistance analyzed by HOMA-IR, and significant reduction in insulin sensitivity was observed (HOMA-IS, FGIR) [Table 2] (p<0.01).
Treatment with G. sylvestre (2 and 4 gm/kg/p.o.) aqueous extract significantly prevented the Dex induced insulin resistance (HOMA-IR). The G. sylvestre (2 and 4 gm/kg/p.o.) extract also improved both HOMA-IS and FGIR [Table 1] (p<0.05). No significant difference was observed between G. sylvestre high dose (4 gm/kg/p.o.) and rosiglitazone (8 mg/kg/p.o.) in improving insulin sensitivity [Table 2].
Discussion
Glucocorticoids are well recognized as the stress hormones. During stress conditions they maintain the homeostasis of glucose metabolism, immune function, cell proliferation and survival. [22,23]. But, long term exposure to excess steroids, even in the absence of starvation becomes maladaptive. [24].
Glucocorticoids reduce the sensitivity of insulin towards the peripheral tissues and diminish insulin action. [25]. The glucocorticoid induced insulin resistance is of multiple pathways, on liver tissue it raises the activity of phosphoenolpyruvate carboxykinase/ glucose-6-phosphatase and promotes gluconeogenesis, in skeletal muscle it promotes muscle wasting and in visceral adipose tissues they promote free fatty acid release. [26,27]. By all these actions they disrupt the glucose homeostasis. Pancreas initially secretes a large quantity of insulin to compensate hyperglycemia and eventually it fails to reduce glucose levels leading to insulin resistance and onset of diabetes. [28].
Dex is a well-known steroid to induce insulin resistance in experimental animals. In a study by Dumas et al, Dex 1.5 mg/ kg in Wistar rats raised glucose, insulin and free fatty acid levels and further induced resistance. [29]. Similarly in another study, Dex 10 mg/kg significantly raised glucose and altered lipid profile. [30]. In the current study also, the use of Dex clearly showed significant raise in glucose and insulin levels. The cellular mechanism responsible for Dex induced resistance is by altering insulin signaling pathways. Previous studies stated that the Dex reduces the expression of Insulin Receptor Substrate-1, Phosphatidiylionosiol-3Kinase and protein kinase B in skeletal muscle, liver and adipose tissues. This inturn leads to the reduced translocation of glucose transporters to cell surface which further leads to hyperglycemia and resistance to insulin action. [31,32]. In the current study, Dex also impaired insulin sensitivity which was apparent by insulin resistance markers.
In the present study, G. sylvestre aqueous extract significantly reduced the elevated glucose and insulin levels. The probable mechanism for blood glucose reduction may be attributed to enhancement of glucose utilization by insulin dependent pathways and promotion of glucose uptake into tissues. [13,33]. Kanetkar et al reported that the active phytochemical constituent Gymnemic acid from the G. sylvestre leaves raises the activity of phosphorylase and reduces the activity of gluconeogenic enzymes and sorbitol dehydrogenase. [13,34]. Further these enzymatic changes might raise the glucose utilization as well as reduction in the release of glucose into circulation.
G. sylvestre also reduces the dietary glucose absorption. EI Shafey et al., reported the probable mechanism may be due to gymnemic acids in the leaves. These molecules reduce the absorption of sugar molecules by filling the receptors located on intestine. This action might be responsible for low blood glucose levels with this extract. [35]. It was also reported that chewing the leaves of G. sylvestre causes loss of taste sensation to sweetness. Thereby it reduces the craving for sweets; this action might be beneficial for diabetics. [35].
Oral glucose tolerance test (OGTT) serves as index to measure the glucose tolerance, but interference of various gastrointestinal factors may act as barriers to diagnose abnormalities in carbohydrate metabolism. [36]. Hence, the present study demonstrated the alterations in carbohydrate metabolism by using intra peritoneal glucose tolerance test (IPGTT). Dex administration significantly raised glucose, insulin values during IPGTT, showing glucose intolerance and resistance to insulin action. Impaired glucose tolerance establishes the diabetes. In the current study Dex by inducing insulin resistance impaired glucose tolerance and further it, induced diabetes. Treatment with G. sylvestre aqueous extract (2 and 4 gm/kg/ p.o.) significantly prevented the glucose intolerance, diabetes and improved sensitivity to insulin action.
HOMA-IR was useful in measuring β-cell function and insulin mediated hepatic glucose output. In the present study, Dex altered insulin release from pancreas and raised glucose release which was evident by rise in circulating blood glucose levels. G. sylvestre administration reduced HOMA-IR levels, indicating the improvement of insulin action on peripheral tissues. Even though the exact mechanism is not clear, G. sylvestre may overcome resistance by enhancing insulin entry into cells. [13]. This action might have reduced the circulating levels of insulin and partially prevented the resistance as observed in the current study. Further, the extract also improved whole body insulin sensitivity evident by fall in HOMA-IS and FGIR levels.
Rosiglitazone is a standard insulin sensitizer drug. It is beneficial in the treatment of insulin resistance and diabetes. However, the major drawback with this drug is cardiovascular complications. [37]. In the present study, we used to compare the insulin sensitizing activity of G. sylvestre extract with rosiglitazone. There was no significant difference between these drugs in improving insulin sensitivity.
Conclusion
To conclude that Gymnema sylvestre extract helpful in the treatment of insulin resistance as shown by reduction in glucose and insulin levels. Furthermore it is evident by reduction of HOMA-IR and improvement in HOMA-IS and FGIR levels. Hence, it can be used as a safer insulin sensitizer drug with minimal side effects. Further identification and isolation of specific phytochemical constituents of this extract responsible for insulin sensitizing activity will be helpful for discovery of newer insulin sensitizer.
Conflict of Interest
All authors disclose that there was no conflict of interest.
REFERENCES
- Vaccarino V, Bremner JD. Stress response and the metabolic syndrome. Cardiology. 2005; 11(Part 2):1
- Sivabalan S, Renuka S, Menon VP. Fat feeding potentiates the diabetogenic effect of dexamethasone in wistar rats. Fat feeding potentiates the diabetogenic effect of dexamethasone in wistar rats. Int Arch Med 2008; 1:7.
- Goundaries JS, Korach-Andre M, Killary K, Argentieri G, Turner O, Laurent D. Effect of dexamethasone on glucose tolerance and fat metabolism in a diet-induced obesity model. Endocrinology 2008; 149:758-766.
- Khan SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 2003; 46:3-19.
- Buse MG. Hexosamines, insulin resistance, and the complications of diabetes: current status. Am J Physiol Endocrinol Metab 2006; 290:E1-E8.
- Biradar KV, Ramesh H, Malipatil M, Bhande RM, Rao KS. Effect of L-Ascorbate on dexamethasone induced experimental insulin resistance-Role of oxidative stress. International journal of pharmaceutical & biological research 2011; 2:643-647.
- Rhee MS, Perianayagam A, Chen P, Youn JH, McDonough AA. Dexamethasone treatment causes resistance to insulin-stimulated cellular potassium uptake in the rat. Am J Physiol Cell Physiol 2004; 287:C1229-1237.
- Dumas JF, Bielicki G, Renou JP, Roussel D, Ducluzeau PH, Malthiery Y, et al. Dexamethasone impairs muscle energetics, studied by 31P NMR,in rats. Diabetologia 2005; 48: 328-335.
- Hans P, Vanthuyne A, Dewandre PY, Brichant JF, Bonhomme V. Blood glucose concentration profile after 10 mg dexamethasone in non-diabetic and type 2 diabetes patients undergoing abdominal surgery. Br J Anaesth 2006; 97:164-170.
- Binnert C, Ruchat S, Nicod N, Tappy L. Dexamethasone-induced insulin resistance shows no gender difference in healthy humans. Diabetes Metab 2004; 30: 321-326.
- Sing B, Saxena A. Surrogate markers of insulin resistance: A review. World J Diabetes 2010; 1:36-47
- Subramaniyan V, Srinivasan P. Gymnema sylvestre – A key for diabetes management – A review. BMR Pharmacology & Toxicology research 2014; 1:1-10
- Kanetkar P, Singhal R, Kamat M. Gymnema sylvestre: A memoir. J Clin Biochem Nutr 2007; 41:77-81.
- Mall GK, Mishra PK, Prakash V. Antidiabetic and hypolipidemic activity of Gymnema sylvestre in alloxan induced diabetic rats. Global J Biotech & Biochem 2009; 4: 37-42.
- Harbone JB. Phytochemical methods: A guide to modern techniques of plant analysis. 2nd ed. Chapmanand Hall, London; 1984:84-88.
- Kokate CK, Purohit AP, Gokhale SB. Pharmacognosy, Nirali Prakashn. 21st edn, Pune; 2002: pp 105-106, 111-113.
- Jamkhande PG, Patil PH, Surana SJ. Evaluation of N-Butanolic fractions of Butea monosperma flowers on dexamethasone induced hyperglycemia and hyperlipidemia in mice. International journal of phyto pharmacy research 2010; 1:5-10.
- Hemanth KV, Nagendra Nayak IM, Shobha HV, Saeed YM, Narendar K, Rajasekhar CH. Dose dependent hepatic and endothelial changes in rats treated with dexamethasone. J Clin Diagn Res 2015: 9: FF08-FF10.
- Sarath BK, Nagendra N, Hebbal GV. Hypoglycemic effect of alcohol extract of Eugenia jambolana seed against dexamethasone induced diabetes in rats. Int J Med Health Sci 2015; 4: 77-81.
- Deushi M, Nomura M, Kawakami A, Haraguchi M, Ito M, Okazaki M, et al. Ezetimibe improves liver steatosis and insulin resistance in obese rat model of metabolic syndrome. FEBS Lett 2007; 581:5664-5670.
- Aref AB, Ahmed OM, Ali LA, Semmler M. Maternal rat diabetes mellitus deleteriously affects insulin sensitivity and beta-cell function in the offspring. J Diabetes Res 2013; 2013.
- Oakley RH, Cidlowski JA. Cellular processing of the glucocorticoid receptor gene and protein: new mechanisms for generating tissue specific actions of glucocorticoids. J Biol Chem 2011; 286:3177-184.
- O’Connor TM, O’Halloran DJ, Shanahan F. The stress response and the hypothalamic-pituitary-adrenal axis: from molecule to melancholia. QJM 2000; 93: 323-333
- Walker BR. Extra-adrenal regeneration of glucocorticoids by 11beta-hydroxysteroid dehydrogenase type 1: physiological regulator and pharmacological target for energy partitioning. Proc Nutr Soc 2007; 66:1-8
- Andrews RC, Walker BR. Glucocorticoids and insulin resistance: old hormones, new targets. Clin Sci (Lnd) 1999; 96: 513-523.
- Pasieka AM, Rafacho A. Impact of glucocorticoid excess on glucose tolerance: clinical and preclinical evidence. Metabolites 2016; 24:1-21
- Rafacho A, Ortsäter H, Nadal A, Quesada I. Glucocorticoid treatment and endocrine pancreas function: Implications for glucose homeostasis, insulin resistance and diabetes. J. Endocrinol 2014; 223: R49–R62.
- Kasuga M. Insulin resistance and pancreatic ß cell failure. J Clin Invest 2006; 116:1756-1760
- Dumas JF, Bielicki G, Renou JP, Roussel D, Ducluzeau PH, Malthiery Y, Simard G, Ritz P. Dexamethasone impairs muscle energetics, studied by 31P NMR, in rats. Diabetologia 2005; 48:328-335.
- Shil D, Mohanty JP, Das T, Bhuyan NR, Uriah T, Saleem TM. Protective role of pitcher of Nepenthes khasiana hook against dexamethazone induced hyperlipidemia and insulin resistance in rats. Int J Res Pharma Sci 2010; 1:195-198.
- Buren J, Liu HX, Jensen J, Eriksson JW. Dexamethasone impairs insulin signaling and glucose transport by depletion of insulin receptor substrate-1, phosphatidylinositol 3-kinase and protein kinase and protein kinase B in primary cultured rat adipocytes. Eur J Endocrinol 2002; 146:419-429
- Saad MJ, Folli F, Kahn JA, Kahn CR. Modulation of insulin receptor, insulin receptor substrate-1, and phosphatidylinositol 3-kinase in liver and muscle of dexamethasone-treated rats. Journal of Clinical Investigation 1993; 92:2065-2072.
- Liu B, Asare-Anane H, Al-Romaiyan A, Huang G, Amiel SA, Jones PM, Persaud SJ. Characterisation of the insulinotropic activity of an aqueous extract of Gymnema sylvestre in mouse ß-cells and human islets of Langerhans. Cell Physiol Bioche 2009; 23:125-132.
- Balamurali KR, Sujiha RR, Harika J, Swapna D, Jagadeeswara RK. Isolation and characterization of gymnemic acid from Gymnema sylvestre R.BR.in control of diabetes. International Journal of Life Science& Pharma Research 2012; 2:L1-L9.
- EI Shafey AA, EI-Ezab MM, Seliem MM, Ouda HH, Ibrahim DS. Effect of Gymnema sylvestre R.Br leaves extract on certain physiological parameters of diabetic rats. Journal of King Saud University – Science 2013; 25:135-141.
- Bowe JE, Franklin ZJ, Hauqe-Evans AC, King AJ, Persaud SJ, Jones PM. Metabolic phenotyping guidelines: assessing glucose homeostasis in rodent models. J Endocrinol 2014; 222:G13-G25.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular diseases. N Engl J Med 2007; 356:2457-2471.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.