Investigate the Inhibitory Effects of Satureja khuzestanica Essential Oil against Housekeeping fabD and exoA Genes of Pseudomonas aeruginosa from Hospital Isolates using RT-PCR Technique
2 Department of Biology, College of Science, Yadegar-e-Imam Khomeini (RAH), Shahr Rey Branch, Islamic Azad University, Tehran, Iran, Email: sayyedeh_M@gmail.com
3 Department of Pharmaceutics, School of Pharmacy, Tehran, Iran, Email: arash_m@yahoo.com
4 Department of Microbiology and Immunology, School of Medicine, Kashan University of Medical Sciences, Kashan, Iran, Email: azadkhaledi@healthcare.com
5 Nursing Department Basic Sciences Faculty, Hamedan Branch, Islamic Azad University, Hamedan, Iran, Email: hosseinvazini@healthcare.com
6 Applied Microbiology Research center and Microbiology Department, Baqiyatallah University of Medical Sciences, Tehran, Iran, Email: esm114@gmail.‘com
Citation: Davoud Esmaeili. Investigate the Inhibitory Effects of Satureja khuzestanica Essential Oil against Housekeeping fabD and exoA Genes of Pseudomonas aeruginosa from Hospital Isolates using RT-PCR Technique. Ann Med Health Sci Res. 2017; 7:246-250
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: Pseudomonas aeruginosa is an opportunistic pathogen which causes a range of infections. Satureja khuzestanica essences have antibacterial effect. Aim: This study aimed to investigate the inhibitory effects of S. khuzestanica essential oil against housekeeping fabD and exo A genes of Pseudomonas aeruginosa from hospital isolates using RT-PCR technique. Material and Methods: The inhibition effect was conducted according to the CLSI 2013 standards and instructions. The minimum inhibitory concentration (MIC) of essence was determined according to CLSI. Isolates were collected of burn patients in the Motahari hospital, Tehran and cultured on proper media and confirmed using microbiology and biochemical. Susceptibility and MIC of S. khuzestanica were determined against P. aeruginosa. Expressions of exoA and Housekeeping fabD genes were evaluated using RT-PCR in presence and lack tests of S. khuzestanica essence. Results: Diameter of inhibition zone of S. khuzestanica essence against P. aeruginosa was 25 mm. MIC of essence was 0.31 μl/ml. Gene expression of exo A under inducing with essential oil of S. khuzestanica reduced but gene expression of housekeeping gene DNA gyraseA not changed before and after exposure. Conclusion: Our results showed that S. Khuzestanica could cause reduction in drug resistance and inhibition of gene expression of exoA in P. aeruginosa. Also, RTPCR is an effective technique for evaluation of gene expression.
Keywords
Pseudomonas aeruginosa; Satureja khuzestanica; ExoA gene
Introduction
Pseudomonas aeruginosa is an opportunistic pathogen that causes a range of infections, including; wound and hospital infections, and infections of respiratory tract [1]. P. aeruginosa is an opportunistic pathogen with ability of infection in both humans and animals. P. aeruginosa is a main agent of bacteremia in patients who received organ transplants and is accountable for approximately 28% of bacteremia cases. Colonization of lungs with this organism is also a common cause of morbidity and mortality in patients who suffered from cystic fibrosis [2]. Owing to the presence of several mechanisms of antimicrobial resistance, the control of this organism in hospitals is often problematic [3].
This micro-organism is one of the most common pathogens in hospitals and causes hospital-acquired infections [4]. The increasing advent of resistant isolates of P. aeruginosa can cause infections among hospitalized patients [5].
P. aeruginosa generates a diversity of extracellular factors which are possibly harmful to ocular tissues. The organism have a potentially ability to produce a number of proteases; (alkaline protease, staphylolytic protease, elastase, and protease IV), a heat-labile and heat-stabile hemolysin, phospholipase C, and toxins (exoenzyme T, -S, and –U, and exotoxin A [ETA].). ETA is a type II secreted 66-kDa A-B toxin, whose function is equal with diphtheria toxin created by Corynebacterium diphtheria. It is bind to the host using the α2-macroglobulin receptor, concurency with its internalization, the enzymatic subunit A is released into the cytoplasm. This subunit transfers adenosine diphosphate (ADP)-ribose of nicotinamide adenine dinucleotide (NAD) to elongation factor(EF)-2 and consequently hinders protein translation [6]. P. aeruginosa has been increasingly recognized for its ability to cause significant hospital-associated with outbreaks of infection, particularly since emerging of multidrug-resistant strains. Outbreaks of multidrug-resistant P. aeruginosa colonization or infection have been reported in urology wards, burn unit, hematology/oncology unit, and adult and neonatal critical care units [7].
Resistance of bacteria to antibiotics has become one of the main public health concerns world-wide, and because of the side effects of conventional antibiotics, further studies have been just concentrated on discover of newly natural antimicrobial agents of herbs, animal and other sources. Herbs are rich in an extensive diversity of secondary metabolites, for example; tannins, terpenoids, which there are some reports about their antimicrobial features [8]. The reasons for propensity to use medicinal plants are; their fairly safe mods, extensive approval by users. Many researchers have reported the antimicrobial, antifungal and antioxidant properties of Satureja khuzestanica essential oils [9].
Satureja khuzestanica Jamzad (SKJ; Marzeh Khuzestani in Persian language, family of Laminaceae and the Nepetoidae subfamily) is found in southern and south western regions of Iran. The plant is characterized with a subshrub, branched stem about 30 cm high, and having the densely leaf, and covered with white hairs [10].
Recently, component of the essential oil of S. Khuzestanica has been characterized. The therapeutic properties of this herb such as; analgesic and antiseptic, antibacterial, antiviral, antifungal have been proven [11]. Until now, no toxicity or adverse effects of this plant was reported, for those derivatives of this plant were candidate as supportive drugs [12].
Aim
So, this study aimed to investigate the inhibitory effect of S. Khuzestanica essential oil against housekeeping fabD and exo A genes of P. aeruginosa from hospital isolates using RT-PCR technique
Materials and Methods
Plant substances
The aerial parts of S. Khuzestanica were collected during May 2013 of Lorestan province, Iran and was identified by the Research Institute of Forests and Rangelands. Then, it was dried and ground into the powder. The prepared powder was held in tight bottles for completely protection of light.
Preparation process of the essential oil
Based on the method recommended by the European Pharmacopeia, dry aerial parts (100g) of S. Khuzestanica were subjected to the hydro distillation for 3 hours, using a Clevenger-type apparatus; then obtained essential oil was dried upon anhydrous sodium sulphate and kept at 4°C for further experiments [13].
Analysis of the essential oil using gas chromatography
Chemical composition of the essential oil and extract were evaluated by gas chromatography. The gas chromatograph (beifen 3420 capillary gas chromatograph) was equipped with BP-5 capillary column (30×0.25 mm ID × 0.25 mm film thickness) and the data were taken under the following conditions: initial temperature 60°C, temperature ramp 3°C for 1 min [14].
Bacterial strains and culture conditions
P. aeruginosa strains were clinically isolated from burn infections in Motahari hospital of Tehran, Iran. For this study, 5 strains were isolated and they cultured on Nutrient agar medium. For identification of isolates, they were cultured on Muller-Hinton agar and confirmed with catalase –oxidase – O/F- arginine de-hydrolase tests and antibiotic resistance.
Susceptibility testing
Antimicrobial activity was investigated by disk diffusion method. The bacterial suspension was brought to the 0.5 McFarland. Then with a sterile of bacterial suspension was taken to inoculate the whole surface of a Muller-Hinton agar. 20 μl of essential oil of S. Khuzestanica was applied on a blank disk and aseptically sited on the inoculated plates. Then, the incubation of plates was performed for 24 hours at 37°C. After that, the inhibition zones were measured. Gentamicin 10 μg was used as a positive control for bacterial inhibition.
Characterize the minimum inhibitory zone
The minimum inhibitory concentration (MIC) was assessed by dilution method in Muller-Hinton broth agar according to the CLSI guidelines. Essential oil of S. Khuzestanica (SKEO) was first diluted in DMSO (dimethylsulfoxyde). Serial Dilutions were carried out with concentrations ranging from 10 μl/ml to 0.019 μl/ml. After 24 hours of incubation at 37°C, from each concentration inoculated on Muller-Hinton agar and incubated in 37°C for 24 hours. After this time, the plates investigated.[15].
Extraction of RNA and RT-PCR performance
P. aeruginosa was cultured on Muller-Hinton agar plates for 24 hours at 37°C. After 24 hours, bacterial isolates were suspended in Muller-Hinton broth and harvested by centrifugation (3000 g, and 10 min) and flow through it. So, P. aeruginosa was cultured on MIC concentration of S. Khuzestanica and incubated at 37°C for 24 hours. After 24 hours, it was harvested by centrifugation (3000xg, 10 min). Then, both of RNAs (before and after adding essence) were extracted from bacteria with Cinapure kit (Cinagen, Iran), and RT-PCR was performed in order to determine the values of mRNA based on the kit direction (Vivantis kit, Cinagen, Iran). Design of oligonucleotide primers for PCR were carried out consistent to the sequences of P. aeruginosa exotoxin A (F; 5ꞌ- TT CGTGGATGAACACCTTGA-3ꞌ; R; 5ꞌ-TGCTGCACTACTCCATGGTC-3ꞌ). Control (fabD gene) was used as a housekeeping gene (F; 5ꞌ- GCTCTTCA GGACCATTCTGG-3ꞌ; R; 5ꞌ- ATCCCTCGCATTCGTCTTC- 3ꞌ). Then, PCR products were analyzed using gel electrophoresis.
Statistical analysis
Data analyzed by SPSS (version 22.00) using chi square minimal statistical significance was considered as p < 0.05 significant.
Results
Analysis of the essential oil using gas chromatography
The chemical compounds of S. Khuzestanica essential oil are summarized in Table 1. The major compound of essential oil was Carvacrol (92%), followed by p-Cymene (3.11%).
| S.no | %c | RIb | RTa | Compound |
|---|---|---|---|---|
| 1 | 0.28 | 935 | 4.081 | α –Pinene |
| 2 | 0.39 | 990 | 5.157 | β –Myrcene |
| 3 | 0.49 | 1016 | 5.77 | α –Terpinene |
| 4 | 3.11 | 1023 | 5.964 | p-Cymene |
| 5 | 0.19 | 1027 | 6.067 | β –Phellandrene |
| 6 | 1.24 | 1056 | 6.828 | ɣ - Terpinene |
| 7 | 0.91 | 1098 | 7.944 | Linalool |
| 8 | 0.35 | 1162 | 9.89 | Borneol |
| 9 | 0.65 | 1173 | 2.26 | Terpinene-4-o1 |
| 10 | 0.19 | 1291 | 13.992 | Thymol |
| 11 | 90.88 | 1296 | 14.164 | Carvacrol |
| 12 | 0.15 | 1413 | 17.757 | (Z)-Caryophyllene |
| 13 | 0.21 | 1502 | 20.464 | β –Bisabolene |
| 14 | 0.18 | 1574 | 22.57 | Caryophyllene oxide |
RTc: Retention time (min), RIb: Retention indices determined on HP-5MS capillary column, %a: calculated from TIC data
Table 1: Chemical compositions (%) of S. khuzestanica essential oil analyzed by GC/MS
Susceptibility testing results
The diameter of inhibition zone in disk diffusion method with S. Khuzestanica essential oil and the minimum inhibitory concentration (MIC) values of S. Khuzestanica essential oil on P. aeruginosa isolates showed that the essential oil had a strong antimicrobial activity against selected isolates. The diameters of inhibition zone (mm) for strains 1, 2, 3, 4, 5 were 25, 23, 26, 24 and 25, respectively. Also, the MIC for all selected strains was 0.31 μl/ml.
RT-PCR results
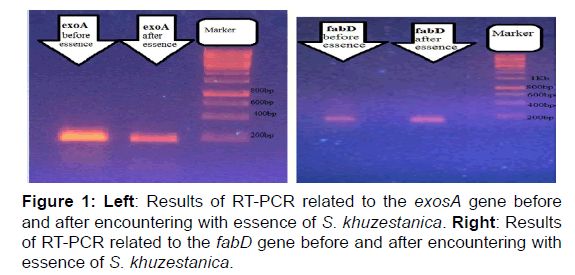
Figure 1 clearly shows that the expression of pathogenic genes exotoxin A (exoA) declined after exposure of bacteria with oil, but expression of fabA after and before exposure did not change.
Discussion
In burns due to the skin destruction as the first line of body defense system, a patient is very susceptible to infections. One of the most important bacteria associated with infection in burn patients is P. aeruginosa. The most important virulence factor of P. aeruginosa is exotoxin A. Exotoxin A in addition to pathogenicity, also plays a role in biofilm formation, and therefore is considered as a factor in antimicrobial resistance [16]. Because of the extreme resistance of P. aeruginosa to antibiotics and disinfectants, find complementary treatment is required.
The genomes of different organisms have many genes that products of some of them are very required at certain times. While the products of some other genes, which are known as the Housekeeping genes in all careers of living cells are needed. fabD is a Housekeeping gene and its product has a role in the biosynthesis of fatty acids, and plays no role in the pathogenesis [17].
In this study, RT-PCR technique has been used to study essential oil effect of S. Khuzestanica on gene expression of exotoxin A. RT-PCR technique is a rapid and highly sensitive method which frequently used to evaluate the expression of the desired gene and is capable to provide semi-quantitative information of gene expression rate [18].
Carvacrol is one of the most effective compounds of S. Khuzestanica which have ability to effect on membranes, proteins and genes, even is able to effect on electron transport chain and even uses as food [19,20].
Qing-huan Yin studied the anti-inflammatory and pre-peptic effects of carvacrol on hepatocellular carcinoma cell line HepG- 2 and revealed that carvacrol had anti liver cancer property. In this study has been investigated the effect of carvacrol on eukaryotic cells and is able to inhibit the growth of cancer cells [21]. A research was conducted by Ultee and et al., presented the bactericidal and bacteriostatic properties of carvacrol such as; ion leakage, loss of turgor pressure, the impact on the synthesis of DNA, decreased metabolic activity [22]. Like mentioned above studies, we found that carvacrol had the antimicrobial properties and its inhibitory concentration on P. aeruginosa (MIC) is 0.31 μg/ml and it became clear that these results are consistent with other researches.
In research conducted by Sara et al. [23] showed that carvacrol possesses inhibitory effect against the production of heat shock protein 60 (HSP60) and flagellum synthesis in E. coli O157: H7. In this study, western blot technique was used [23]. The study was conducted by Ultee and colleagues showed that carvacrol had bactericidal and bacteriostatic properties as well as some properties such as leakage of essential ions, loss of turgor pressure, and the impact on the synthesis of DNA, decreased metabolic activity [24]. In line with this study, we also found that the S. Khuzestanica had a bacteriostatic and bactericidal activity.
In a study conducted by Kim et al. [25] examined the antibacterial effects of carvacrol on Salmonella typhimurium and strains resistant to rifampicin. They showed that carvacrol had potent antibacterial effects against both strains [25]. We also studied the antibacterial effects of essential oil of S. Khuzestanica, which has a high concentration of carvacrol, the results showed this plant has potent antibacterial effect on the bacterium. Amiri et al. studied the anti-bacterial effect of essential oil of S. Khuzestanica on infectious bacteria of respiratory tract [20]. Like their study, our study showed that the essential oil of S. Khuzestanica have very large bactericidal effects on P. aeruginosa. Carvacrol like the phenolic compounds acts by increasing the secretion of the bacterial cell membrane to H +, K +, and reduces the ATP and impact on the DNA and RNA and consequently death of bacteria [26]. In study of Ghodrati and colleagues in 2015 surveyed the antimicrobial activity of S. Khuzestanica against Candida albicans, E. coli, S. epidermidis and Bacillus cereus by both disk diffusion and micro dilution methods using plant extracts of S. Khuzestanica, in which, the most its activity was against yeast Candida albicans and lowest was observed against S. epidermidis and E. coli with MIC= 0.1- 0.19 μg/ml that indicated that the MIC of extract was lower than MIC of essential oil [27]. Zerrin and colleagues studied the carvacrol effect on cancer cells H-RAS and N-RAS, and the results showed carvacrol had cytotoxic effects on the cells in dose and appropriate time [28]. The results showed that cells and genes with high activity show greater sensitivity to antimicrobial agents. Our results also suggest that bacterial cells are more sensitive in logarithmic a growth phase. Llana-Ruiz-Cabello and et al. [29] investigated the cytotoxic effects of carvacrol on cervical cancer cells (HELA cells and SITRA) also, cytotoxic effects of carvacrol were determined by MTT and LDH technique [29]. The results of our study with other studies also concluded that the carvacrol with less concentration is effective on eukaryotic cells. In addition, cancer cells because proliferate faster, their DNA, RNA, membrane; cytochrome oxidase and protein synthesis are more sensitive to carvacrol than normal cells. So we conclude that the natural cells show more resistance to carvacrol. Our study also showed that prokaryotic cells for inhibition of gene expression are needed to more concentrations of carvacrol, because prokaryotes have a cell wall and outer membrane, the influence of carvacrol takes place slower [29]. Their results showed that the cells and genes with high activity have a greater sensitivity to antimicrobial agents. Our results also suggest that bacterial cells are more sensitive in logarithmic growth phase. In 2012, A study examined anti-proliferative effects of carvacrol against liver cancer cells (HEPG-2) and results showed that carvacrol inhibits cell proliferation by inducing apoptosis, [30] also, their results revealed that the cells and genes with high activity have a greater sensitivity to antimicrobial agents. Chou, Chiang and et al. in 2013 examined effects of carvacrol for the release of calcium, cell survival, apoptosis and formation of channel in cancer cells of human mouth, results showed that carvacrol causes calcium release, formation of channels in oral cancer cells and eventually causes apoptosis through activate caspase-3 [31]. Real Time PCR technique has been used to evaluate the expression of eukaryotic and prokaryotic times and times, also this technique has been used to study gene analysis of benzevat and salysilat in two strains of P. aeruginosa, but we used of RT-PCR technique as semi-quantitative. The preference and excellence of Real time- RT-PCR is that shows the gene expression as semi-quantitative, quietly. But we used dilution method and semi quantative RT-PCR in order to show expression of exoA as quantatively.
One of the innovations of this research is that for the first time, RT-PCR technique was used for evaluate the effect of S. Khuzestanica on gene expression of exotoxin A from P. aeruginosa.
It be noted, in this study, the initial tests have confirmed that this substance have inhibitory effect on P. aeruginosa. And we know that in in vivo, the break point of drugs is important. So with bactericidal or bacteriostatic substances can assess inhibitory effect on gene expression. In this study, was exposed P. aeruginosa with MIC concentration of S. Khuzestanica and gene expression level were measured by Q-PCR. And then in next step, it can be offered as a treatment for patients with burns infection.
Conclusion
Our results showed that S. Khuzestanica could cause reduction in drug resistance and inhibition of gene expression of exoA in P. aeruginosa. Also, RT-PCR is an effective technique for evaluation of gene expression.
Acknowledgement
We would like to express our sincere thanks for our colleagues in Motahari hospital for their helps.
Conflict of Interest
None
Author Contribution
Daoud Esmaeili and Arash Mahboubi contributed to design, and prepare a draft of the manuscript; Azad Khaledi, Asieh Abbasi and Sayyedeh Mahdokht Maddah contributed to do clinical experiments and data analysis; All authors accepted the final version of this manuscript.
REFERENCES
- Driscoll JA, Brody SL, Kollef MH. The epidemiology, pathogenesis and treatment of Pseudomonas aeruginosa infections. Drugs. 2007;67:351-368.
- Lyczak JB, Cannon CL, Pier GB. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes and Infection. 2000;2:1051-1060.
- Hasanien YAM. Studies on incidence and prevention of nosocomial infection of urinary tract endoscopies by different antimicrobial agents: Zagazig University, Egypt. 2010.
- Peleg AY, Hooper DC. Hospital-acquired infections due to gram-negative bacteria. N Engl J Med. 2010;362:1804-1813.
- Obritsch MD, Fish DN, MacLaren R, Jung R. Nosocomial infections due to multidrug resistant Pseudomonas aeruginosa: epidemiology and treatment options. Pharmacotherapy: J Human Pharmacol Drug Therap. 2005;25:1353-1364.
- Willcox MD. Pseudomonas aeruginosa infection and inflammation during contact lens wear: A review. Optometry & Vision Science. 2007;84:273-278.
- Hota S, Hirji Z, Stockton K, Lemieux C, Dedier H, Wolfaardt G, et al. Outbreak of multidrug-resistant Pseudomonas aeruginosa colonization and infection secondary to imperfect intensive care unit room design. Infection Control & Hospital Epidemiology. 2009;30:25-33.
- Perumal Samy R, Gopalakrishnakone P. Therapeutic potential of plants as anti-microbials for drug discovery. J Evid Based Complementary Altern Med 2010;7:283-294.
- Nostro A, Roccaro AS, Bisignano G, Marino A, Cannatelli MA, Pizzimenti FC, et al. Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. J Med Microbiol 2007;56:519-523.
- Assaei R, Mostafavi-Pour Z, Pajouhi N, Omrani GHR, Sepehrimanesh M, Zal F. Effects of essential oil of Satureja khuzestanica on the oxidative stress in experimental hyperthyroid male rat. Veterinary Research Forum; 2015: Faculty of Veterinary Medicine, Urmia University, Urmia, Iran.
- Pirbalouti AG, Moalem E. Variation in antibacterial activity of different ecotypes of Satureja khuzestanica Jamzad, as an Iranian endemic plant. Ind J Tradit Know. 2013;12:623-629.
- Safarnavadeh T, Rastegarpanah M. Antioxidants and infertility treatment, the role of Satureja Khuzestanica: A mini-systematic review. Iran J Reprod Med. 2011;9:61.
- Nikbin M, Kazemipour N, Maghsoodlou MT, Valizadeh J, Sepehrimanesh M, Davarimanesh A. Mineral elements and essential oil contents of Scutellaria luteo-caerulea Bornm. & Snit. Avicenna J Phytomedicine. 2014;4:182.
- Mardani M, Afra SM, Tanideh N, Andisheh Tadbir A, Modarresi F, Koohi Hosseinabadi O, et al. Hydroalcoholic extract of Carum carvi L. in oral mucositis: A clinical trial in male golden hamsters. Oral Diseases. 2016;22:39-45.
- Vandenbossche I, Vaneechoutte M, Vandevenne M, De Baere T, Verschraegen G. Susceptibility testing of fluconazole by the NCCLS broth macrodilution method, E-test, and disk diffusion for application in the routine laboratory. J Clini Microbiol. 2002;40:918-921.
- Cao H, Baldini RL, Rahme LG. Common mechanisms for pathogens of plants and animals. Annual Rev Phytopathol. 2001;39:259-284.
- Savli H, Karadenizli A, Kolayli F, Gundes S, Ozbek U, Vahaboglu H. Expression stability of six housekeeping genes: a proposal for resistance gene quantification studies of Pseudomonas aeruginosa by real-time quantitative RT-PCR. J Med Microbiol. 2003;52:403-408.
- Saleh-Lakha S, Miller M, Campbell RG, Schneider K, Elahimanesh P, Hart MM, et al. Microbial gene expression in soil: methods, applications and challenges. J Microbiol Methods. 2005;63:1-19.
- Abdollahi M, Salehnia A, Mortazavi SHR, Ebrahimi M, Shafiee A, Fouladian F, et al. Antioxidant, antidiabetic, antihyperlipidemic, reproduction stimulatory properties and safety of essential oil of Satureja Khuzestanica in rat in vivo: A toxicopharmacological study. Medical Science Monitor. 2003;9:BR331-BR5.
- Amiri M, Esmaeili D, Sahlehnia A, Ariana M, Alam F, Beiranvand H. Study of antibacterial effects of Satureja essence against some common nosocomial pathogenic bacteria. Int J Curr Microbiol App Sci. 2013;2:249-254.
- Hutchison ML, Govan JR. Pathogenicity of microbes associated with cystic fibrosis. Microbes and Infection. 1999;1:1005-1014.
- Burt SA, van der Zee R, Koets AP, de Graaff AM, van Knapen F, Gaastra W, et al. Carvacrol induces heat shock protein 60 and inhibits synthesis of flagellin in Escherichia coli O157: H7. Appl. Environ. Microbiol. 2007;73:4484-4490.
- Burt SA, Vlielander R, Haagsman HP, Veldhuizen EJ. Increase in activity of essential oil components carvacrol and thymol against Escherichia coli O157: H7 by addition of food stabilizers. Journal of Food Protection®. 2005;68:919-926.
- Ultee A, Bennik M, Moezelaar R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl Environ Microbiol. 2002;68:1561-1568.
- Kim J, Marshall M, Cornell J, JF III P, Wei C. Antibacterial activity of carvacrol, citral, and geraniol against Salmonella typhimurium in culture medium and on fish cubes. J Food Sci. 1995;60:1364-1368.
- Evans K, Adewoye L, Poole K. MexR Repressor of the mexAB-oprMMultidrug Efflux Operon of Pseudomonas aeruginosa: Identification of MexR Binding Sites in the mexA-mexRIntergenic Region. J Bacteriol. 2001;183:807-812.
- Ghodrati L, Alizadeh A, Ketabchi S. Essential oil constituents and antimicrobial activities of Iranian Satureja khuzistanica Jamzad. International Journal of Biosciences (IJB). 2015;6:249-257.
- Akalin G, Incesu Z. The effects of carvacrol on apoptosis of H-ras and N-ras transformed cell lines. Turk J Pharm Sci. 2011;8:105-116.
- Llana-Ruiz-Cabello M, Gutiérrez-Praena D, Pichardo S, Moreno FJ, Bermúdez JM, Aucejo S, et al. Cytotoxicity and morphological effects induced by carvacrol and thymol on the human cell line Caco-2. Food and Chemical Toxicology. 2014;64:281-290.
- Yin QH, Yan FX, Zu XY, Wu YH, Wu XP, Liao MC, et al. Anti-proliferative and pro-apoptotic effect of carvacrol on human hepatocellular carcinoma cell line HepG-2. Cytotechnology. 2012;64:43-51.
- Liang WZ, Chou CT, Lu T, Chi CC, Tseng LL, Pan CC, et al. The mechanism of carvacrol-evoked (Ca2+). i rises and non-Ca2+-triggered cell death in OC2 human oral cancer cells. Toxicology. 2013;303:152-161.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.