Trilogy-Function Thiadiazole-Triazole-Pyridine Derivatives as Anticancer Activity
Received: 29-Dec-2021, Manuscript No. AMHSR-22-50118; Editor assigned: 31-Dec-2021, Pre QC No. AMHSR-22-50118(PQ); Accepted Date: Jan 31, 2022 ; Reviewed: 18-Jan-2022 QC No. AMHSR-22-50118; Revised: 21-Jan-2022, Manuscript No. AMHSR-22-50118(R); Published: 31-Jan-2022, DOI: 10.54608.annalsmedical.2022.25
Citation: Hammed AD, et al. Trilogy-Function Thiadiazole-Triazole-Pyridine Derivatives as Anticancer Activity. Ann Med Health Sci Res. 2022;12:49-59.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
1-Methyl-4-(6-(subsitutedphenyl)-(1,2,4)triazolo(3,4-b)(1,3,4)thiadiazol-3-yl)- alkylpyridinium was synthesized in this study. The 4-Amino-5-pyridin-4-yl-4H-(1,2,4) triazole-3-thiol was combined with substituted phenacyl bromide in a condensation reaction to produce 4-Amino-5-pyridin-4-yl-4H-(1,2,4)triazole-3-thiol. 1-Methyl-4- (6-(substitutedphenyl)-(1,2,4)triazolo(3,4-b)(1,3,4)thiadiazol-3-yl)pyridinium was alkylated with three distinct alkyl bromides. The anticancer effects of the produced triazolo (3,4-b)(1,3,4)thaidiazole pyridine derivatives were investigated using FTIR and 1H-NMR, and some compounds in the series showed promising anti-cancer activity when compared to standard medications.
Keywords
Triazole; Thiadiazole; Antibacterial; Antifungal
Introduction
Cancer is a serious disease that is still a major medical concern around the world. It is the second most frequent sickness after cardiovascular diseases. As a result, the development of seriously strong and potent novel antineoplastic medications is one of the most certain goals of modern therapeutic science. The science of 1,2,4-triazoles and their intertwined heterocyclic subordinates has gotten a lot of attention because of its planned and significant organic applicability. Numerous 1,2,4-triazoles, for example, have been incorporated into a wide range of therapeutically intriguing antibacterial pharmaceutical up-and-comers, [1,2] reducing, [3] reducing pain [4] and anticancer workouts. [5] Mercapto 1,2,4-triazoles are also quite useful in preparative natural science as helpful intermediates in the design of several triazolothiadiazines and triazolothiadiazines, despite their major natural applications. The amino and mercapto groups provide immediate nucleophilic sites for the mixture of consolidated heterocyclic rings. [6] The triazolothiadiazoles and triazolothiadiazines, which have a broad natural profile, have now evolved into important heterocyclic platforms. [7,8] The term MCF-7 refers to the Michigan Cancer Foundation-7, a detroit-based organization where herbert soule and coworkers established the cell line in 1973. [9,10] MCF7 is a sort of malignant growth beginning from bosom tissue, most regularly from the internal covering of milk channels or the lobules that stock the pipes with milk. [11] MCF-7 cells are valuable for in vitro bosom disease contemplates on the grounds that the cell line has held a few ideal attributes specific to the mammary Epithelium. These incorporate the capacity for MCF-7 cells to deal with estrogen, as estradiol, through estrogen receptors in the cell cytoplasm. This makes the MCF-7 cell line an Estrogen Receptor (ER) positive control cell line. [12] Apoptosis is a crucial component of cell programming that leads to cell death in long-lived vertebrates. [13] Because it assumes a critical role in the development and maintenance of homeostasis. [14] It is the process of removing excess or damaged cells from the body. Furthermore, the balance between current cell formation and cell passage is what maintains the appropriate number of cells in a tissue. [15] Apoptosis is a vital life measure for all living things. Managed cell passage plays an important role in various natural cycles that occur in all species, such as cell turnover, tissue homeostasis, embryogenesis, acceptance and support of invulnerable resilience, and improvement of the sensory system, and endocrine ward tissue decay. [16]
Experimental Study
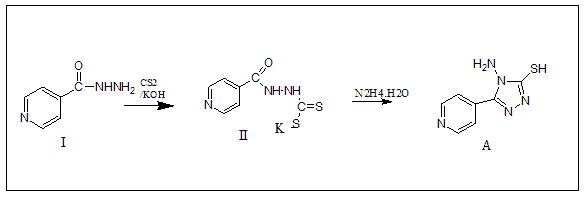
1-Synthetic Methods of 4-Amino-5-pyridin-4-yl-4H-(1,2,4)triazole-3-thiol(A)
At ambient temperature, isonicotinic acid hydrazide (13.7 g, 0.1 mole) was dissolved in pure ethanol (200 mL) containing potassium hydroxide (11.2 g, 0.1 mole) and carbon disulphide (13.17 mL, 0.21 mole) was added in parts to a 500 mL round bottom flask. After 16 hours of agitation, the mixture was diluted with dry diethyl ether (100 mL). The substance was then filtered and vacuum dried at 65?-70? The potassium dithiocarbazinate salt was produced in approximately quantitative yields as described above. With stirring, hydrazine hydrate (0.01 mole, 99 percent) was gradually added to potassium dithiocarbazinate salt dissolved in water (100 mL) and refluxed over water bath until H2S was produced.
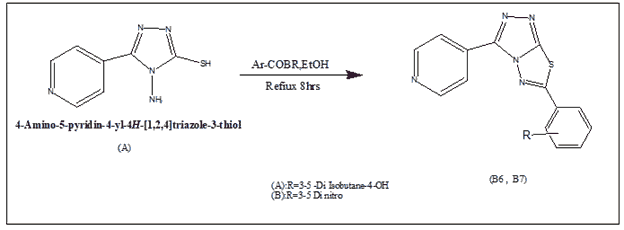
Synthesis of 1-Methyl-4-(6-phenyl-(1,2,4)triazolo(3,4-b)(1,3,4)thiadiazol-3-yl)-pyridinium(B1-B2)
In 100% ethanol, a 0.01 mol mixture of 4-amino-5-pyridin-4-yl-4H-(1,2,4)triazole-3-thiol and the corresponding phenacyl bromide (0.012 mol) was heated under reflux for 8 hours. The reaction liquid was placed onto crushed ice, and the pH was adjusted with Na2CO3. Filtered, washed with water, dried, and crystallized from ethanol, the solid product was obtained.
Preparation of N-(Ethyl, propyl, pentyl) pyridinium bromide derivatives (C1-C6)
In a round-bottomed flask equipped with a reflux condenser, a solution of (10 mmol) of (B1-B2) and (10 mmol) of n-Ethyl bromide, n-Propyl bromide, and n-Pentyl bromide in 2 ml of 100% ethanol was allowed to stand overnight at room temperature. The mixture was then cooked for 10 hrs at reflux temperature. The pyridinium chloride derivative solution is then dried to remove the solvent and refined by washing with diethyl ether.
Preparation of N-(Ethyl, propyl, pentyl) pyridinium bromide derivatives (C1-C6)
In a round-bottomed flask equipped with a reflux condenser, a solution of (10 mmol) of (B1-B2) and (10 mmol) of n-Ethyl bromide, n-Propyl bromide, and n-Pentyl bromide in 2 ml of 100% ethanol was allowed to stand overnight at room temperature. The mixture was then cooked for 10 hours at reflux temperature. The pyridinium chloride derivative solution is then dried to remove the solvent and refined by washing with diethyl ether.
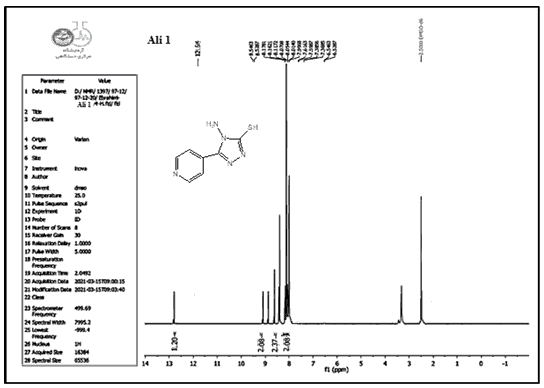
Compounds (A) also characterized using 1HNMR. Figures 1-9 show the 1HNMR spectrum of compound (IV) (DMSO-d6, δ in ppm): 7.43-7.62 (d, 4H, arom. H) for the pyridine ring, 12.93 (s, 2H, SH), 9.81 (s, 2H, N-H ).
1-Methyl-4-(6-phenyl-(1,2,4)triazolo(3,4-b) The reaction of 4-amino-5-(pyridin-4-yl)-4H-1,2,4-triazole-3-thiol (A) with two substituted phenacyl bromide yielded (1,3,4)thiadiazol-3-yl)-pyridinium (B1-B2).
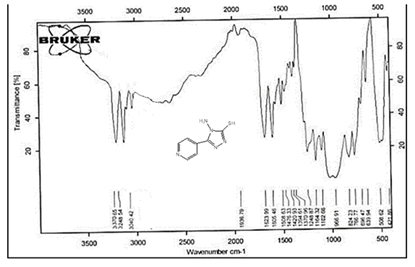
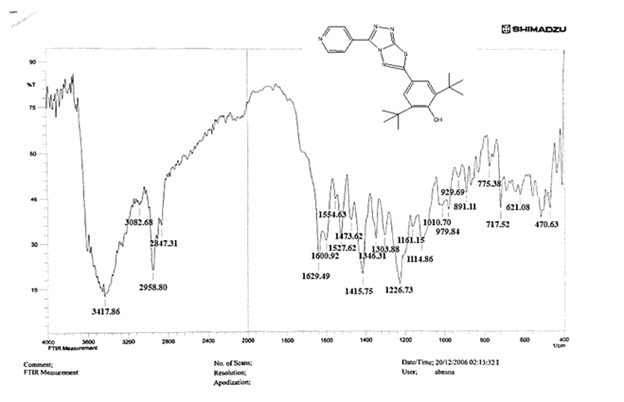
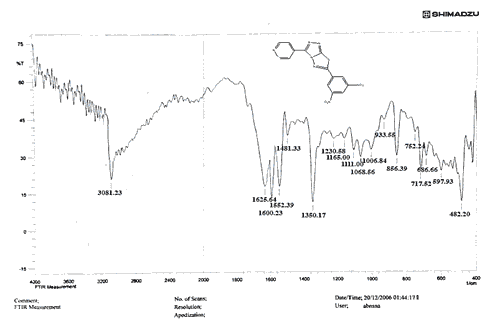
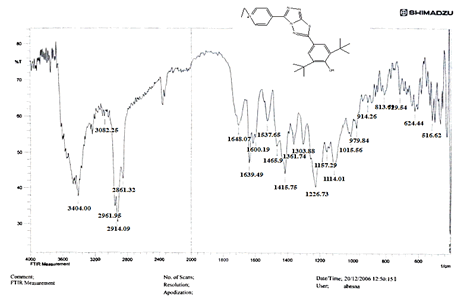
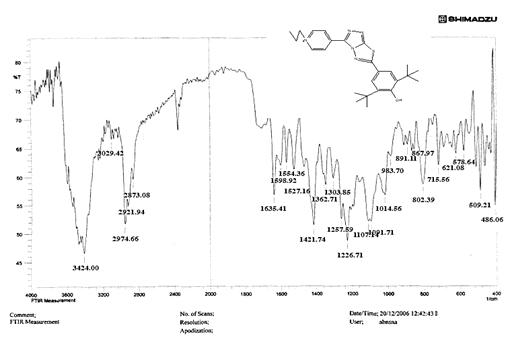
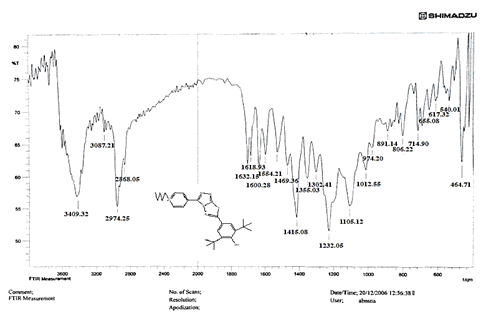
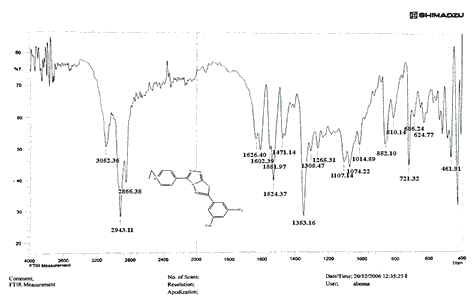
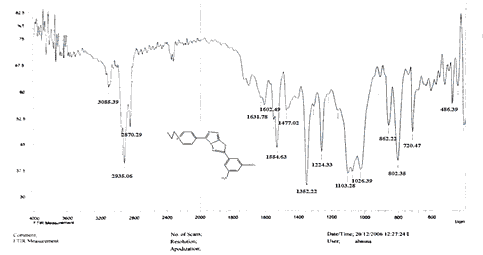
The NH2 and S-H stretching bands vanished from the FTIR spectra of compounds (B1-B2). New bands at (3417, 3082, 2958, 2847, 1629, 1600, 1527, and 1346) cm-1 could be assigned to O–H, aromatic C–H, aliphatic, C=N, C=C, and NO2 stretching bands, respectively, in the FTIR spectrum of compound (B1), which was synthesized from reaction compound (A) with 3,5-diisobutyl-4-hydroxy phenacyl bromide. The FTIR spectra of compound (B2), which was produced by reacting compound (A) with 3,5-dinitro phenacyl bromide, exhibits bands at (3081, 1625, 166, 1552, and 1350 cm-1 that may be ascribed to C–H aromatic, C=N, C=C, and asymmetrical and symmetrical NO2 stretching bands, respectively.
Alkylation of the pyridine nitrogen atom with ethyl, propyl, and pentyl bromides yielded N-(Ethyl, Propyl, Pentyl) pyridinium bromide derivatives (C1-C6).
The intensity of C–H aliphatic asymmetrical and symmetrical stretching increases in the FTIR spectra of compounds (C1-C3) synthesized from reaction compound (B1) with ethyl, propyl, and pentyl bromide at (2961, 2974) and (2861, 2873, 2858) cm-1, respectively.
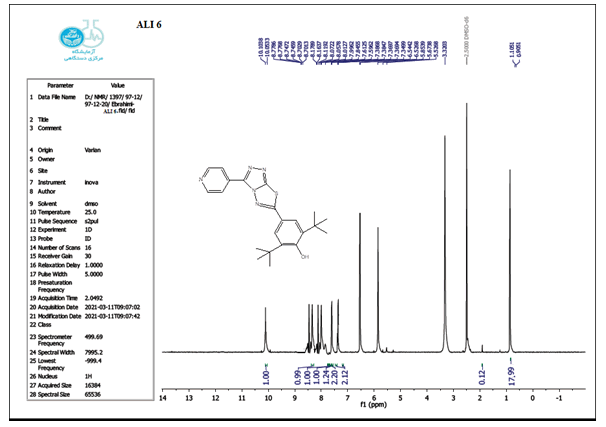
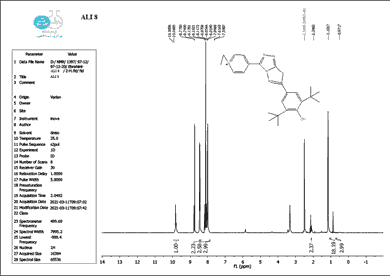
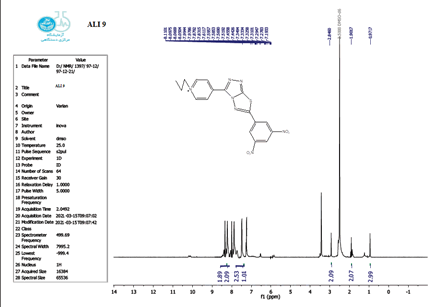
The structure of the produced compounds was also studied using the 1HNMR spectroscopic method. The 1HNMR spectra of chemical C1 is shown in Figures 10-13. Singlet at 10.10 for hydroxy group (d6-DMSO, ppm). The six protons of the phenyl and pyridine rings are responsible for two signals at 7.5-8.7. The eighteen protons of the tert. butyl group are assigned to the signal at 1.10. The signal triplet at 0.97 (3 H) and quartet signal at 2.2 (2 H) on the 1HNMR may be referred to the ethyl group.
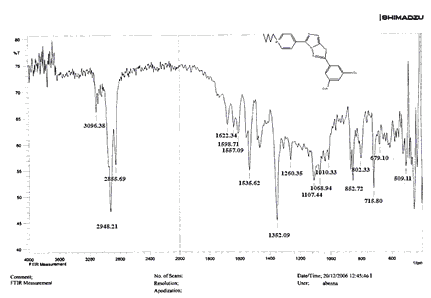
Compounds (C4-C6) produced from reaction compound (B2) with ethyl, propyl, and pentyl bromide display novel bands of C-H aliphatic asymmetrical and symmetrical stretching at (2943, 2935,) and (2866, 2870,) cm-1, respectively, in their FTIR spectra.
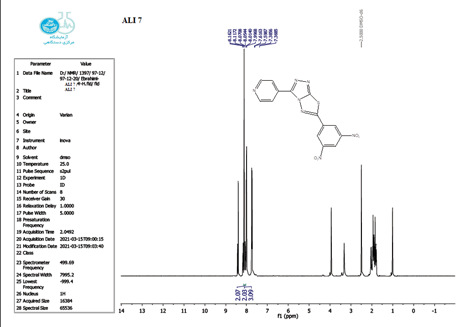
The 1 HNMR spectrum of chemical C4 is shown in Figure 14 (d6-DMSO, ppm), the six protons of the phenyl and pyridine rings are responsible for two signals at 7.5-8.7. The eighteen protons of the tert butyl group are assigned to the signal at 1.10. The signal triplet at 0.97 (3 H) and quartet signal at 2.2 (2 H) on the 1HNMR may be referred to the ethyl group.
Anticancer Activity
On two types of cancer cell lines, MCF-7 and WRL68, the MTT assay was utilized to find chemicals with lethal effects. After employing varied amounts of each chemical on cancer cell lines, this experiment was used to detect cell viability. .
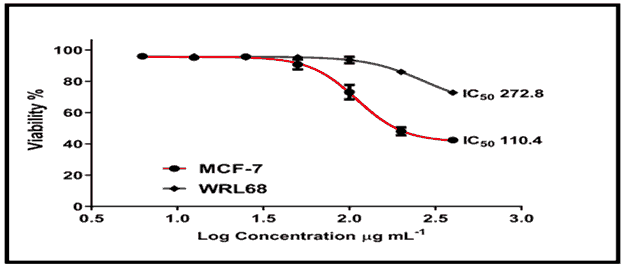
The cytotoxic effect of chemical (C2) revealed that treating MCF-7 cells at concentrations ranging from 12.5 to 400 g/ml for 24 hours resulted in a considerable loss of cell viability, which increased in a dose-dependent pattern, reaching up to 70% death at 200 g/ml with an IC50 of 110.4/ml [Figure 15, Tables 1 and 2]. The treatment of WRL68 cells with compound (C2), on the other hand, proved to be less affected when the same concentration range was used, with a detected IC50 of 272.8/ml [Figure 15].
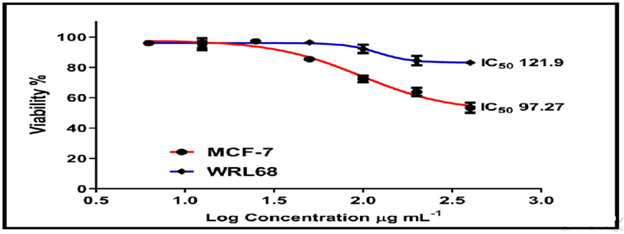
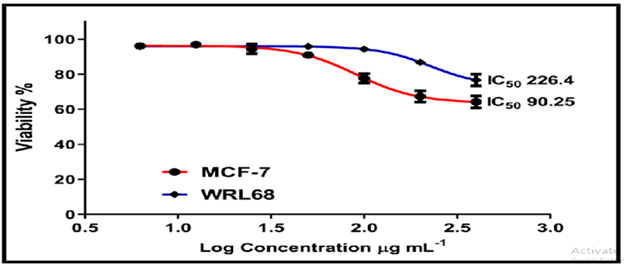
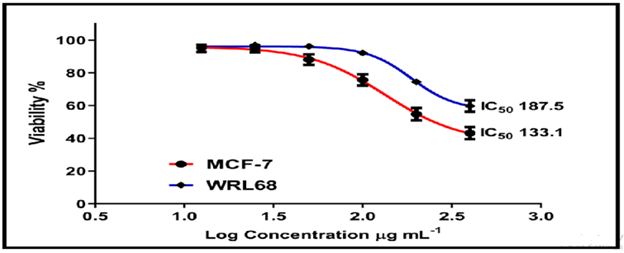
On the normal cell line MCF-7, the other chemicals in Figures 16-18 showed weak to moderate cytotoxicity, with IC50 values ranging from 190.0 to 90.02 g/mL. The IC50 for WRL-68 was found to be between 267.9 and 121.9 g/mL.
Figure 19 shows that when the concentration of Z officinal's extract was 200 g/mL, the viable count of MCF-7 cells decreased compared to untreated cells. The drop in cell counts was substantially different from control in cells dealing with twenty five, fifty, one hundred, and two hundred g/mL of Z. officinal's compound, but in different ranges. This result strongly suggests This suggests that landfills are effective in killing cells, as evidenced by the MTT assay, which revealed that the drop in cell count was dosage dependant, with the most significant reduction occurring after administration of high concentrations (P 0.001) dosage (200 g/mL).
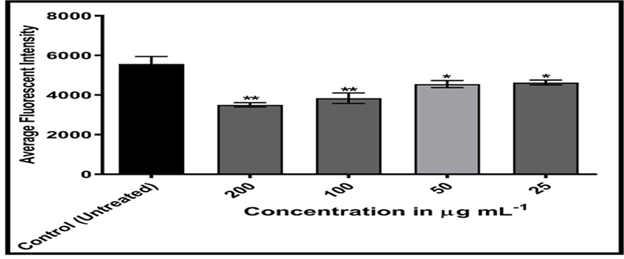
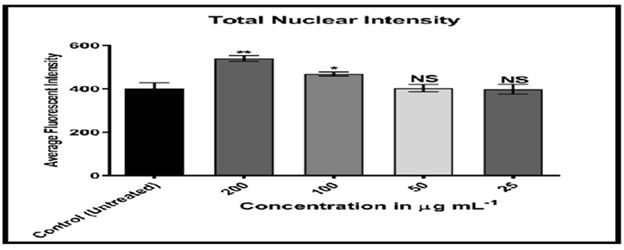
Figures 20-24 shows, on the other hand, that the intensity of MCF-7 cell membrane permeability (green) increased dose-dependently, with a considerable rise after exposure to 100 and 200 of the substance. Officinal g/mL. The fluorescence intensity of compound and doxorubicin (powerful anti-tumor agent) was revealed in comparison to untreated cells, with doxorubicin in comparison, the nuclear intensity increased greatly when exposed to 200 g/mL of compound solution. Chemotherapy embeds itself in DNA molecules, generating twice the amount of DNA damage.
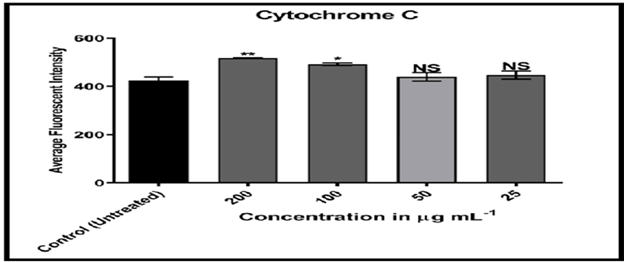
Cytochrome C plays an important function in apoptosis and can be released into the cytoplasm from mitochondria. [17] MCF-7 cells treated with compound (C2) solution displayed high nuclear cytochrome C staining when compared to doxorubicin, indicating that treatment of breast cancer cells with compound (C2) mimics cytochrome C from mitochondria to cytoplasm.
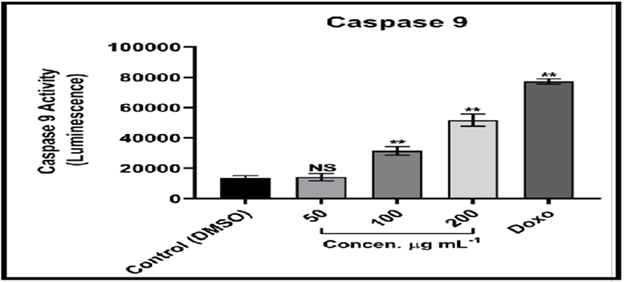
The Caspase-Glo 9 test is a homogenous luminescence assay that evaluates caspase-9 activity in human cells. [18] Caspase-9 is a major activator in the intrinsic apoptotic cascade. The findings suggest that MCF-7 cells treated with compound (C2) solution undergo apoptosis through breaking cell membrane and cytochrome C release, as these two events are linked to Caspase activation. [19] Caspase 9 and 3 activation are instances of initiators and effectors, respectively; it is also implicated in the intrinsic pathway of mitochondrial dependency. The intrinsic pathway is primarily regulated by mitochondria, which is not only the site where anti-apoptotic proteins and pro-apoptotic proteins interact and determine cell fate, but also the place where anti-apoptotic proteins and pro-apoptotic proteins interact and define cell fate. However, it is also the source of the signal that triggers Caspase activation via a variety of pathways. [20-22]
Conclusion
This work involve the synthesis new trilogy compounds containing thiadiazole triazole-pyridine rings. The structures of the synthesized compounds were investigated by FTIR and 1HNMR. The MTT assay was used to examine the cytotoxic effect of compounds on two types of tumor cell lines, MCF-7 and WRL68. The cytotoxic effect of chemical (C2) revealed that treating MCF-7 cells at concentrations ranging from 12.5 to 400 g/ml for 24 hours resulted in a considerable loss of cell viability, which increased in a dose-dependent pattern, reaching up to 70% death at 200 g/ml with an IC50 of 110.4/ml. The treatment of WRL68 cells with compound (C2), on the other hand, proved to be less affected when the same concentration range was used, with a detected IC50 of 272.8/ml
Acknowledgments
The authors would like to thank the Department of Chemistry at Al-Nahrain University for their help and cooperation throughout this research.
References
- Isloor AM, Kalluraya B and Shetty P. Regioselective reaction: Synthesis, characterization and pharmacological studies of some new Mannich bases derived from 1,2,4-triazoles. Eur J Med Chem. 2009;44:3784-3787. [Crossref], [Google Scholar]
- Alhusam S. Clinical conditions and risk factors of acinetobacter baumannii producing metallo beta-lactamases among hospitalized patients. Journal of Scientific Research in Medical and Biological Sciences. 2021;2:11-17. [Crossref], [Google Scholar]
- Prakash O, Aneja DK, Hussain K, Lohan P, Ranjans P. Synthesis and biological evaluation of dihydroindeno and indeno (1,2-e)(1,2,4)triazolo (3,4-b)(1,3,4)thiadiazines as antimicrobial agents. Eur J Med Chem. 2011;46:5065-5073. [Crossref], [Google Scholar], [Indexed]
- Vikrant S, Amey JP, Damle SR. Synthesis and antibacterial activity of some novel bis-1,2,4-triazolo(3,4-b)-1,3,4-thiadiazoles and bis-4-thiazolidinone derivatives from terephthalic dihydrazide. Eur J Med Chem. 2009;44:5112-5116. [Crossref], [Google Scholar], [Indexed]
- Mathew V, Keshavayya J, Vaidya VP, Gilesa D. Studies on synthesis and pharmacological activities of 3,6-disubstituted-1,2,4-triazolo(3,4-b)-1,3,4-thiadiazoles and their dihydro analogues. Eur J Med Chem. 2007;42:823-840. [Crossref], [Google Scholar], [Indexed]
- Padmavathi V, Reddy GS, Padmaja A, Kondaiah P, Shazia A. Synthesis, antimicrobial and cytotoxic activities of 1,3,4-oxadiazoles, 1,3,4-thiadiazoles and 1,2,4-triazoles. Eur J Med Chem. 2009;44:2106-2112. [Crossref], [Google Scholar], [Indexed]
- Husain A, Rashid M, Anees MS, Siddiqui A, Mishra R. Benzimidazole clubbed with triazolo-thiadiazoles and triazolo-thiadiazines: New anticancer agents. Eur J Med Chem. 2013;62:785-798. [Crossref], [Google Scholar], [Indexed]
- Sumangala V, Poojary B, Chidananda N, Arulmoli T, Shenoy S. Facile synthesis, cytotoxic and antimicrobial activity studies of a new group of 6-aryl-3-(4-(methylsulfonyl)benzyl)-7H-(1,2,4)triazolo(3,4-b)(1,3,4)thiadiazines. Eur J Med Chem. 2012;pp:5459-64. [Crossref], [Google Scholar], [Indexed]
- Khana A, Ibrara, Abbas N. Triazolothiadiazoles and triazolothiadiazines-biologically attractive scaffolds. Eur J Med Chem. 2013;63:854-868. [Crossref], [Google Scholar]
- Harris RE, Alshafie GA, Abou-Issa H, Seibert K. Chemoprevention of breast cancer in rats by celecoxib, a cyclooxygenase 2 inhibitor. Cancer Res. 2000;60:2101-2103. [Crossref], [Google Scholar], [Indexed]
- Soule HD, Vazquez J, Long A, Albert S, Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst. 1973;51:1409-1416. [Crossref], [Google Scholar], [Indexed]
- Lacroix M, Leclercq G. Relevance of breast cancer cell lines as models for breast tumours: An update. Breast Cancer Res Treat. 2004;83:249-289. [Crossref], [Google Scholar], [Indexed]
- Hassan M, Watari H, AbuAlmaaty A, Ohba Y, Sakuragi N. Apoptosis and molecular targeting therapy in cancer. Bio Med Research International. 2014. [Crossref], [Google Scholar], [Indexed]
- Tamiru Y, Abebe N, Kebede A. Review on mechanisms of regulating apoptosis in animal cells. W J Biomed Pharm Sci. 2017;2:33-38. [Crossref], [Google Scholar]
- Hadi DM, Jber NR. Synthesis and spectroscopic characterization of bis-swallow tailed mesogen. Int J Sci Res. 2017;6:1909-1915. [Crossref], [Google Scholar]
- Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: Structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem. 1998;68:383-424. [Crossref], [Google Scholar]
- Loong HH, Yeo W. Microtubule-targeting agents in oncology and therapeutic potential in hepatocellular carcinoma. Onco Targets Therapy. 2014;7:575. [Crossref], [Google Scholar], [Indexed]
- Abdul HAM, Farouk HA, Fahmy MS. Efficacy of hayman suture in the treatment of atonic postpartum hemorrhage. JMBSR. 2021; 2:38-43. [Crossref], [Google Scholar], [Indexed]
- Choi YJ, Gurunathan S, Kim JH. Graphene oxide-silver nanocomposite enhances cytotoxic and apoptotic potential of salinomycin in human ovarian cancer stem cells (OvCSCs): A novel approach for cancer therapy. Int J Mol Sci. 2018;19:710. [Crossref], [Google Scholar], [Indexed]




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.